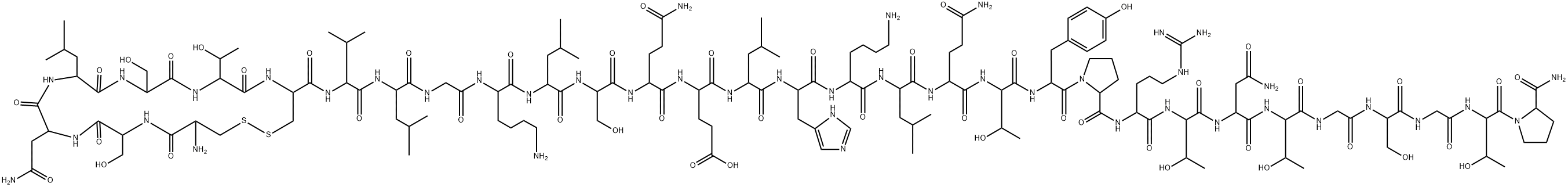
Carbetocin
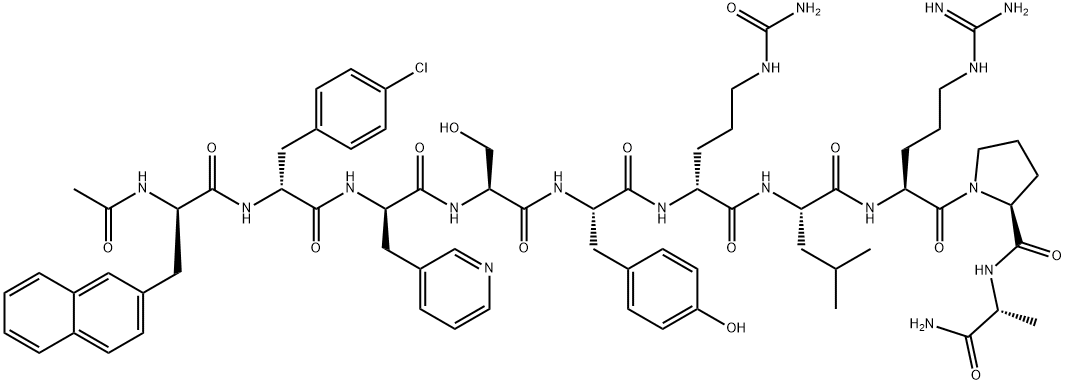
Synonym(s):N-(4-Mercapto-1-oxobutyl)-O-methyl-L-tyrosyl-L-isoleucyl-L-glutaminyl-L-asparaginyl-L-cysteinyl-L-prolyl-L-leucyl-glycinamidecyclic (1-5)-thioether acetate
- CAS NO.:37025-55-1
- Empirical Formula: C45H69N11O12S
- Molecular Weight: 988.17
- MDL number: MFCD01076600
- EINECS: 253-312-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-04 13:44:46
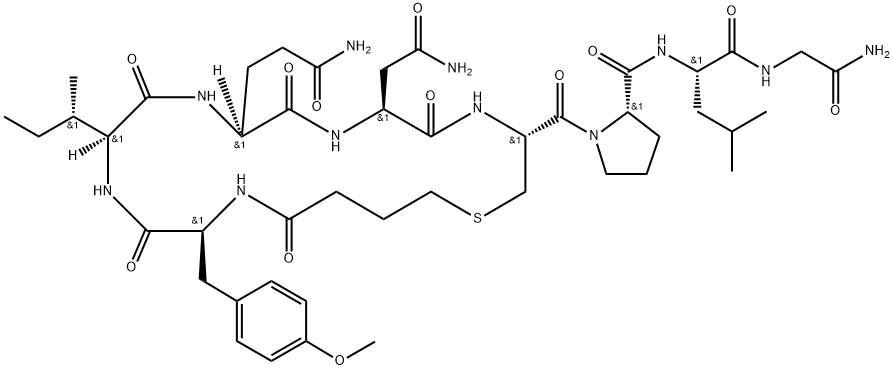
What is Carbetocin?
Absorption
Bioavailability is 80% following intramuscular injection.
Description
Carbetocin is an alternative synthetic oxytocin-receptor agonist available in Canada, the United Kingdom, and other countries,but not the United States. A large multinational randomized noninferiority trial (N=29,645) found that carbetocin was noninferior to oxytocin for prevention of postpartum hemorrhage and need for additional uterotonic agents after vaginal delivery. Carbetocin has a longer duration of action than oxytocin, eliminating the need for prolonged infusion.
The Uses of Carbetocin
Carbetocin is an obstetric drug used to control postpartum hemorrhaging.
A new synthetic analog of oxytocin, carbetocin, is long acting and also has a rapid onset of action. It is an oxytocin receptor agonist and also stimulates milk let down due to its action on oxytocin receptors of myoepithelial cells.
Background
Carbetocin is a drug used to control postpartum hemorrhage, bleeding after giving birth. It is an analogue of oxytocin, and its action is similar to that of oxytocin -- it causes contraction of the uterus.
Indications
Used to control postpartum hemorrhage and bleeding after giving birth.
Definition
ChEBI: Oxytocin in which the hydrogen on the phenolic hydroxy group is substituted by methyl, the amino group on the cysteine residue is substituted by hydrogen, and the sulfur of the cysteine residue is replaced by a methylene group. A synthetic carba-analogue o oxytocin, it is used to control bleeding after giving birth. Like oxytocin, it causes contraction of the uterus.
Biochem/physiol Actions
Carbetocin is a potent agonist of the oxytocin receptor, with improved in vivo stability over oxytocin.
Pharmacokinetics
Carbetocin is a drug used to control postpartum hemorrhage, bleeding after giving birth. It is sold under the trade name Duratocin. It is an analogue of oxytocin, and its action is similar to that of oxytocin; it causes contraction of the uterus.
Metabolism
Not Available
storage
Store at -20°C
Properties of Carbetocin
| Melting point: | 140 - 160°C |
| alpha | D -69.0° (c = 0.25 in 1M acetic acid) |
| Boiling point: | 1477.9±65.0 °C(Predicted) |
| Density | 1.218±0.06 g/cm3(Predicted) |
| storage temp. | -15°C
|
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 13.07±0.70(Predicted) |
| form | powder |
| color | white to beige |
| Water Solubility | Soluble to 29.65 mg/ml in water |
| Stability: | Hygroscopic |
Safety information for Carbetocin
Computed Descriptors for Carbetocin
| InChIKey | NSTRIRCPWQHTIA-FOILTOEQNA-N |
Carbetocin manufacturer
Piramal Pharma Solutions
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 37025-55-1 Carbetocin 99%View Details
37025-55-1 Carbetocin 99%View Details
37025-55-1 -
 37025-55-1 99%View Details
37025-55-1 99%View Details
37025-55-1 -
 Carbetocin 98%View Details
Carbetocin 98%View Details
37025-55-1 -
 Carbetocin acetate CAS 37025-55-1View Details
Carbetocin acetate CAS 37025-55-1View Details
37025-55-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1