Fluconazole
Synonym(s):2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol;Fluconazole
- CAS NO.:86386-73-4
- Empirical Formula: C13H12F2N6O
- Molecular Weight: 306.27
- MDL number: MFCD00274549
- EINECS: 627-806-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 15:44:27
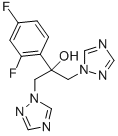
What is Fluconazole?
Description
Fluconazole is a prescription antifungal medication approved by the FDA for the treatment of a variety of fungal diseases, including vaginal candidiasis, oropharyngeal and esophageal candidiasis, peritonitis, and systemic Candida infections, including candidemia, disseminated candidiasis, pneumonia, and cryptococcal meningitis.
Chemical properties
Fluconazole-D4 is White to Off-White Solid
Originator
Pfizer (United Kingdom)
Background
Fluconazole, commonly known as Diflucan, is an antifungal drug used for the treatment of both systemic and superficial fungal infections in a variety of tissues. It was initially approved by the FDA in 1990. This drug is an azole antifungal, in the same drug family as ketoconazole and itraconazole. Fluconazole has many advantages over the other antifungal drugs including the option of oral administration. The side effect profile of this drug is minimal. It has been demonstrated as an efficacious treatment for vaginal yeast infections in one single dose.
Indications
Fluconazole can be administered in the treatment of the following fungal infections:
1) Vaginal yeast infections caused by Candida
2) Systemic Candida infections
3) Both esophageal and oropharyngeal candidiasis
4) Cryptococcal meningitis
5) UTI (urinary tract infection) by Candida
6) Peritonitis (inflammation of the peritoneum) caused by Candida
Definition
ChEBI: Fluconazole is a member of the class of triazoles that is propan-2-ol substituted at position 1 and 3 by 1H-1,2,4-triazol-1-yl groups and at position 2 by a 2,4-difluorophenyl group. It is an antifungal drug used for the treatment of mucosal candidiasis and for systemic infections including systemic candidiasis, coccidioidomycosis, and cryptococcosis. It has a role as a P450 inhibitor, an environmental contaminant and a xenobiotic. It is a difluorobenzene, a conazole antifungal drug, a triazole antifungal drug and a tertiary alcohol. It is functionally related to a 1,3-difluorobenzene. It derives from a hydride of a 1H-1,2,4-triazole.
Manufacturing Process
141.1 g of aluminum trichloride was first added to 86 ml of DFB and 77 ml of chloroacetyl chloride was then added to the mixture, which was allowed to
react at 60°C for 3 hours. After the reaction mixture had cooled down, 500 g
of cold water was added. The mixture was stirred for about 20 min and then
filtered to afford about 158.5 g of 2-chloro-2',4'-difluoroacetophenone in solid
form (91% yield).
A solution of 158.5 g of 2-chloro-2',4'-difluoroacetophenone and 88.8 g of 4-
amino-4H-1,2,4-triazole in 1,600 ml of cyanomethane was heated at reflux for
16 hours, cooled down, and filtered. The solid thus obtained was then washed
with 500 ml of ethyl ether once to afford 2-(1H-1,2,4-triazol-1-yl)-2',4'-
difluoroacetophenone salt.
The crude product obtained was dissolved in 1,320 ml of 1.5 N hydrochloric
acid. To the solution thus obtained, an aqueous solution (330 ml) of sodium
nitrite (58.2 g) was dropwise added and the mixture was allowed to react for
30 min. Aqueous ammonium was then used to adjust the reaction mixture to
a neutral pH. The solid was precipitated and filtered to afford 159 g of 2-(1H-
1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (yield about 80%), which had a
water content of about 10%.
4 g of 4-amino-4H-1,2,4-triazole, 57.87 g of potassium hydroxide, 118 g of
trimethyl sulfoxonium iodide, and 100 g of 2-(1H-1,2,4-triazol-l-yl)-2',4'-
difluoroacetophenone were dissolved in 1,600 ml of water. The solution was
heated at 70°C to react for 16 hours. Upon the completion of the reaction, the
solution was adjusted with 4 N hydrochloric acid to a neutral pH and then
extracted with acetyl acetate. The organic layer was collected, dried with 30 g
of anhydrous calcium dichloride, decolorized with 15 g of active charcoal, and
finally filtered off solid residues. The filtrate was concentrated to afford 99.3 g
of the crude product (yield 72%). The crude product was further recrystallized
from 500 ml of a solvent mixture of acetyl acetate and n-hexane (2:1) to
afford 66.3 g of the Fluconazole in the form of white solid (yield 48%).
brand name
Diflucan (Pfizer).
Antimicrobial activity
The spectrum is limited, but includes most Candida spp., Cryptococcus spp., dermatophytes and dimorphic fungi (Blast. dermatitidis, Coccidioides spp., Hist. capsulatum and Paracoccidioides brasiliensis). Strains of C. krusei appear to be insensitive.
Acquired resistance
Resistant strains of C. albicans have been isolated from AIDS patients given long-term treatment for oral or esophageal candidosis. Strains of C. glabrata frequently become resistant during short courses of treatment. There are a few reports of fluconazole-resistant strains of Cryp. neoformans recovered from AIDS patients with relapsed meningitis. Most, but not all, C. albicans and C. glabrata strains resistant to fluconazole are cross-resistant to other azoles.
General Description
Fluconazole is used to treat adult neutropenic patients with invasive candidiasis (IC).
Pharmaceutical Applications
A synthetic bis(triazole) available for oral or parenteral administration. A prodrug formulation, fosfluconazole, is available for intravenous use in Japan.
Biological Activity
Triazole antifungal agent. Effective against Candida strains in vitro and in vivo .
Biochem/physiol Actions
Fluconazole is an antifungal agent. It is highly selective inhibitor of fungal cytochrome P-450 sterol C-14 α-demethyllation. Fluconazole is a potent inhibitor of CYP2C9. Fluconazole interferes with fungal ergosterol synthesis and downregulates the metallothionein gene.
Pharmacokinetics
Fluconazole has been demonstrated to show fungistatic activity against the majority of strains of the following microorganisms, curing fungal infections:
Candida albicans, Candida glabrata (Many strains are intermediately susceptible), Candida parapsilosis, Candida tropicalis, Cryptococcus neoformans
This is achieved through steroidal inhibition in fungal cells, interfering with cell wall synthesis and growth as well as cell adhesion, thereby treating fungal infections and their symptoms.
The fungistatic activity of fluconazole has also been shown in normal and immunocompromised animal models with both systemic and intracranial fungal infections caused by Cryptococcus neoformans and for systemic infections caused by Candida albicans. It is important to note that resistant organisms have been found against various strains of organisms treated with fluconazole. This further substantiates the need to perform susceptibility testing when fluconazole is considered as an antifungal therapy.
A note on steroidal effects of fluconazole
There has been some concern that fluconazole may interfere with and inactivate human steroids/hormones due to the inhibition of hepatic cytochrome enzymes. Fluconazole has demonstrated to be more selective for fungal cytochrome P-450 enzymes than for a variety of mammalian cytochrome P-450 enzymes. Fluconazole 50 mg administered daily for up to 28 days in individuals of reproductive age has been show to have no effect on testosterone plasma concentrations of males and plasma concentrations of steroids in females. A 200-400 mg dose of fluconazole showed no clinically relevant effect on steroid levels or on ACTH-stimulated steroid response in healthy males, in one clinical study mentioned on the European Medicines Agency label. Other studies have shown no significant effects of fluconazole on steroid levels, further confirming these data.
Pharmacology
It has good oral absorption, is well tolerated, and is preferentially taken up in keratinized tissues, reaching concentrations up to 50 times that in plasma. This allows for once-weekly dosing in most cases.
Clinical Use
Fluconazole is very effective in the treatment of infections
with most Candida spp. Thrush in the end-stage
AIDS patient, often refractory to nystatin, clotrimazole,
and ketoconazole, can usually be suppressed with oral
fluconazole.AIDS patients with esophageal candidiasis
also usually respond to fluconazole.
Fluconazole may be an acceptable alternative to
amphotericin B in the initial treatment of mild cryptococcal
meningitis, and it has been shown to be superior
to amphotericin B in the long-term prevention of relapsing
meningitis (such patients require lifelong treatment.).
Coccidioidal meningitis, previously treated with
both intravenous and intrathecal amphotericin B, appears
to respond at least as well to prolonged oral fluconazole
therapy. Aspergillosis, mucormycosis, and
pseudallescheriasis do not respond to fluconazole treatment.
Sporotrichosis, histoplasmosis, and blastomycosis
appear to be better treated with itraconazole, although
fluconazole does appear to have significant activity
against these dimorphic fungi.
Mechanism of action
Fluconazole (Diflucan) is an azole antifungal medication that works by stopping the fungus from being able to make a protective covering. This causes the fungus to not grow or survive.
Side Effects
Adverse drug reactions associated with fluconazole therapy include
Common (≥1% of patients): rash, headache, dizziness, nausea, vomiting, abdominal pain, diarrhea, and/or elevated liver enzymes
Infrequent (0.1–1% of patients): anorexia, fatigue, constipation
Rare (<0.1% of patients): oliguria, hypokalaemia, paraesthesia, seizures, alopecia, Stevens–Johnson syndrome, thrombocytopenia, other blood dyscrasias, serious hepatotoxicity including liver failure, anaphylactic/anaphylactoid reactions Very rare: prolonged QT interval, torsades de pointes
Toxicity
Acute oral toxicity (LD50): 1271 mg/kg (rat)
Overdose information
Fluconazole overdoses have been associated with hallucination and paranoia, sometimes in combination. In cases of overdose, employ supportive treatment. Gastric lavage may be necessary. Other modalities such as forced diuresis or hemodialysis may also be used.
A note on liver toxicity
The FDA label warns that this drug carries a risk of hepatotoxicity. Rare but serious cases of serious hepatic toxicity have been reported, especially in patients with serious underlying medical conditions using fluconazole. This group of patients has an increased risk of fatality when using fluconazole. In patients with existing liver dysfunction, use caution during fluconazole therapy. Those who are found to have abnormal liver function tests during therapy should be carefully monitored for the development of increasingly severe injury to the liver. Fluconazole should be stopped if its use is likely to be the underlying cause of liver injury, and medical attention should be sought. Fluconazole induced hepatotoxicity is usually reversible.
Carcinogenesis, mutagenesis, and impairment of fertility
Fluconazole demonstrated no evidence of carcinogenic risk in mice and rats treated orally for 24 months at doses equivalent to approximately 2-7 time the recommended human dose). Male rats given fluconazole at doses equivalent to supratherapeutic human doses showed an increased incidence of hepatocellular adenomas. Cytogenetic studies in vivo and in vitro demonstrated no sign of chromosomal mutation. The significance of these findings for humans is unknown.
Use in pregnancy
There are no sufficient and well-controlled studies of fluconazole use in pregnant women. Available human data do not show an increased risk of congenital anomalies after pregnant women were treated with standard doses (<200 mg/day) of fluconazole, either in a single dose or multiple doses in the first trimester did not appear to impact the fetus negatively. Several case reports describe rare but striking congenital anomalies observed in infants who were exposed to fluconazole at high doses reaching 400-800 mg/day, primarily in the first trimester of pregnancy. Similar findings were observed in animal studies. If this drug is administered during pregnancy, or if the patient becomes pregnant while taking fluconazole, the risk should be discussed thoroughly.
Use in nursing
Fluconazole is secreted in breastmilk at high concentrations. Exercise caution if this drug is used during nursing.
Veterinary Drugs and Treatments
Fluconazole may have use in veterinary medicine in the treatment of systemic mycoses, including cryptococcal meningitis, blastomycosis, and histoplasmosis. It may also be useful for superficial candidiasis or dermatophytosis. Because of the drug’s unique pharmacokinetic qualities, it is probably more useful in treating CNS infections or fungal urinary tract infections than other azole derivatives. Fluconazole does not have appreciable effects (unlike ketoconazole) on hormone synthesis and may have fewer side effects than ketoconazole in small animals.
Drug interactions
Potentially hazardous interactions with other drugs Aminophylline: concentration of aminophylline possibly increased. Analgesics: increases concentration of celecoxib - halve celecoxib dose; concentration of flurbiprofen, ibuprofen and methadone increased; increases concentration of parecoxib - reduce parecoxib dose; inhibits metabolism of alfentanil; concentration of fentanyl possibly increased. Anti-arrhythmics: avoid concomitant use with amiodarone due to risk of QT prolongation. Antibacterials: avoid with erythromycin; increases rifabutin levels - reduce dose; metabolism accelerated by rifampicin; concentration of bedaquiline possibly increased - avoid if fluconazole for >14 days. Anticoagulants: potentiates effect of coumarins. Antidepressants: avoid concomitant use with reboxetine; concentration of amitriptyline and nortriptyline increased. Antidiabetics: possibly enhances hypoglycaemic effect of nateglinide; increases concentration of sulphonylureas. Antiepileptics: increases fosphenytoin and phenytoin levels; possibly increased carbamazepine concentration. Antimalarials: avoid concomitant administration with artemether/lumefantrine and piperaquine with artenimol. Antipsychotics: increased risk of ventricular arrhythmias with pimozide - avoid concomitant use; possibly increases lurasidone concentration; possibly increased quetiapine levels - reduce dose of quetiapine. Antivirals: increases nevirapine, ritonavir, tipranavir and zidovudine levels, and possibly saquinavir: concentration of simeprevir possibly increased - avoid. Anxiolytics and hypnotics: increases diazepam and midazolam levels. Avanafil: concentration of avanafil possibly increased. Bosentan: increased bosentan levels - avoid concomitant use. Ciclosporin: increases blood/serum ciclosporin levels. Clopidogrel: possibly reduced antiplatelet effect. Cytotoxics: possibly increased side effects of cyclophosphamide; concentration of bosutinib and possibly olaparib increased - avoid or reduce dose of bosutinib; possibly increases ibrutinib concentration - reduce ibrutinib dose; reduce dose of ruxolitinib. Dapoxetine: reduce dose of dapoxetine. Diuretics: increased eplerenone levels - avoid concomitant use; concentration of fluconazole increased by hydrochlorothiazide. Ergot alkaloids: increased risk of ergotism - avoid concomitant use. Guanfacine: possibly increased guanfacine dose - halve dose of guanfacine. Ivabradine: increased ivabradine levels - reduce initial dose. Ivacaftor: increased concentration of ivacaftor. Lipid-lowering drugs: possibly increased risk of myopathy with atorvastatin or simvastatin; concentration of fluvastatin increased possibly increased risk of myopathy; avoid with lomitapide. Retinoids: possibly increased risk of tretinoin toxicity. Sirolimus: may increase sirolimus concentration. Tacrolimus: increases blood/serum tacrolimus levels. Theophylline: possibly increases theophylline levels.
Metabolism
Fluconazole is metabolized minimally in the liver. Fluconazole is an inhibitor of CYP2C9, CYP3A4 and CYP2C19. Two metabolites were detected in the urine of healthy volunteers taking a 50 mg radiolabeled dose of fluconazole; a glucuronidated metabolite on the hydroxyl moiety (6.5%) and a fluconazole N-oxide metabolite (2%). The same study indicated that no signs of metabolic cleavage of fluconazole were observed, suggesting a difference in metabolism when compared to other agents in the same drug class, which are heavily metabolized in the liver.
Storage
Store at +4°C
Properties of Fluconazole
| Melting point: | 138-140°C |
| Boiling point: | 579.8±60.0 °C(Predicted) |
| Density | 1.05 |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: 5 mg/mL |
| form | solid |
| pka | pKa 1.76±0.1(H2O t=24 I=0.1(NaCl)) (Uncertain) |
| color | White to Off-White |
| Water Solubility | 1g/L(temperature not stated) |
| Merck | 14,4122 |
| InChI | InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 |
| CAS DataBase Reference | 86386-73-4(CAS DataBase Reference) |
Safety information for Fluconazole
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Fluconazole
| InChIKey | RFHAOTPXVQNOHP-UHFFFAOYSA-N |
| SMILES | C1(C=CC(F)=CC=1F)C(O)(CN1N=CN=C1)CN1N=CN=C1 |
Fluconazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 FLUCONAZOLE 99%View Details
FLUCONAZOLE 99%View Details -
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 FLUCONAZOLE 99%View Details
FLUCONAZOLE 99%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details -
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details
