Posaconazole
Synonym(s):;Posaconazole solution;Sch 56592
- CAS NO.:171228-49-2
- Empirical Formula: C37H42F2N8O4
- Molecular Weight: 700.78
- MDL number: MFCD00941162
- EINECS: 682-747-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
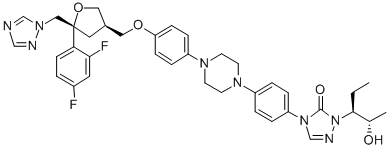
What is Posaconazole?
Absorption
Posaconazole is absorbed with a median Tmax of approximately 3 to 5 hours.
Toxicity
During the clinical trials, some patients received posaconazole up to 1600 mg/day with no adverse events noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg BID for 3 days. No related adverse events were noted by the investigator.
Description
Posaconazole, launched in the UK, is the newest member of the azole class of antifungal agents to reach the market. It is indicated for the treatment and prophylaxis of a range of invasive fungal infections, including aspergillosis,fusariosis, chromoblastomycosis, mycetoma, and coccidiomycosis in patients who are refractory to, or intolerant of, standard therapy with amphotericin B and/or itraconazole. In the US, it is approved for the prophylaxis of invasive Aspergillus and Candida infections in patients 13 years of age who are at high risk of developing these infections due to being severely immunocompromised. Additionally, it is approved for the treatment of oropharyngeal candidiasis. Posaconazole has an expanded spectrum of activity over other members of the azole antifungals. In addition to potent activity against refractory cases of aspergillosis and fluconazole-resistant Candida, it demonstrates activity against Zygomycetes.
Chemical properties
White Solid
Originator
Schering-Plough (US)
The Uses of Posaconazole
Posaconazole is a sterol C14ɑ demethylase inhibitor with an IC50 of 0.25 nM
The Uses of Posaconazole
Orally active triazole antifungal.
The Uses of Posaconazole
Posaconazole has been used:
- in antifungal susceptibility testing of Aspergillus terreus
- as a lanosterol?14α-demethylase (CYP51)-specific inhibitor to study its effects on membrane permeability in Candida albicans cells
- to study its effects on promastigotes
Background
Posaconazole is a triazole antifungal drug that is used to treat invasive infections by Candida species and Aspergillus species in severely immunocompromised patients.
Indications
Posaconazole is prescribed for the prophylaxis of invasive Aspergillus and Candida infections in patients aged 13 years and older who face a high risk of developing these infections due to severe immunocompromise. This includes individuals undergoing procedures such as hematopoietic stem cell transplant (HSCT) with graft-versus-host disease (GVHD) or those with hematologic malignancies experiencing prolonged neutropenia from chemotherapy. Additionally, it is indicated for the treatment of oropharyngeal candidiasis, including cases refractory to itraconazole and/or fluconazole. Posaconazole serves as an alternative treatment for invasive aspergillosis, Fusarium infections, and zygomycosis in patients intolerant of or with diseases refractory to other antifungals, offering a therapeutic option for challenging fungal infections.
What are the applications of Application
Posaconazole is a triazole anti-fungal
Definition
ChEBI: An N-arylpiperazine that consists of piperazine carrying two 4-substituted phenyl groups at positions 1 and 4. A triazole antifungal drug.
brand name
Noxafil
Antimicrobial activity
The spectrum includes dimorphic fungi (Blast. dermatitidis, Coccidioides spp., Hist. capsulatum, Pen. marneffei, and Spor. schenckii), molds (Aspergillus spp., Mucor spp., Rhizomucor spp. and Rhizopus spp.), some dematiaceous fungi and yeasts (Candida spp. and Cryptococcus spp.).
General Description
Technetium (99mTc) exametazime is a mixture of unstablelipophilic enantiomers that rapidly cross the blood-brain barrierand is trapped in the tissues. The proposed trapping mechanismfor localization includes reduction by glutathione. Asimilar diffusion and trapping process occurs with autologouslymphocytes in vitro.
Exametazime is also known as hexamethylpropyleneamineoxime or HMPAO. The radiolabeled complex is indicated for cerebral perfusion in stroke, but is most commonlyused for the radiolabeling of autologous leukocytesas an adjunct in the localization of intra-abdominal infectionand inflammatory bowel disease.
Each kit includes several components: (a) reaction vialscontaining a mixture of exametazime, stannous chloride,and sodium chloride; (b) vials of 1% methylene blue; (c)vials of phosphate buffer in 0.9% NaCl; and (d) 0.45-μm syringefilters. Product preparation depends on the intendeduse.
Pharmaceutical Applications
A synthetic triazole available for oral administration.
Biochem/physiol Actions
Posaconazole is a highly potent broadspectrum antifungal agent against the yeast infection caused especially by Candida sp. It blocks the growth of fungi by inhibiting the enzyme?lanosterol?14α-demethylase (CYP51). In contrast to other antifungal azoles, posaconazole has been reported not to induce the efflux pump mechanism. Posaconazole exhibits antichagasic effects against different strains of Trypanosoma cruzi causing Chagas disease.
Pharmacokinetics
Posaconazole is an antifungal agent structurally related to itraconazole. It is a drug derived from itraconzaole through the replacement of the chlorine substituents with flourine in the phenyl ring, as well as hydroxylation of the triazolone side chain. These modifications enhance the potency and spectrum of activity of the drug. Posaconazole can be either fungicial or fungistatic in action.
Pharmacokinetics
Cmax 200 mg oral: 0.5 mg/L after 4 h
Plasma half-life: 35 h
Volume of distribution: 1774 L
Plasma protein binding: >98%
Absorption
Oral absorption is slow. Absorption from the gastrointestinal tract is improved if the drug is given with a high-fat meal. Blood concentrations increase in proportion to dosage up to 800 mg.
Distribution
It is extensively distributed into body tissues.
Metabolism and excretion
It is not as extensively metabolized by the hepatic cytochrome P450 system as other triazole antifungals. More than 70% of an administered dose is eliminated in the feces, predominantly as unchanged drug. The remainder is excreted as glucuronidated derivatives in the urine. Posaconazole is a substrate for intestinal P-glycoprotein,an adenosine triphosphate-dependent plasma membrane transporter responsible for drug efflux from cells. Multiple peaks in blood concentrations have been observed, suggesting that effluxed drug is reabsorbed into the systemic circulation.
Clinical Use
Invasive aspergillosis
Fusarium infection
Chromoblastomycosis and mycetoma
Coccidioidomycosis
Oropharyngeal candidosis
Prophylaxis of invasive fungal infections in patients at serious risk
With the exception of oropharyngeal candidosis and prophylaxis,
use is presently restricted to patients with disease that
is refractory to other antifungal drugs, or who are intolerant
to them.
Side Effects
It is generally well tolerated even for long periods. Unwanted effects include gastrointestinal discomfort and mild to moderate, transient abnormalities of liver enzymes. Rare side effects include cholestasis and hepatic failure.
Synthesis
Several routes to the synthesis
of posaconazole have been published in the literature. The most likely route to large scale synthesis uses
convergent synthesis of a key chiral THF subunit 101 and
aryl piperazine amine 102 followed by introduction of the
triazole subunit at the end.The readily
accessible allyl alcohol 94 was brominated
(PBr3) to give bromide 95 which was alkylated with sodium
diethylmalonate and the resulting diester was reduced with
NaBH4/LiCl, to give the key diol 97 in very good yields.
After scanning many hydrolases to desymmetrize the diol via
selective acylation, hydrolase SP 435 was found to be suitable. Thus reaction of the diol 97 in the presence of SP
435 with vinyl acetate in acetonitrile gave monoacetate 98 in
greater than 90% yield. Iodine mediated cyclization of the
monoacetate 98 with iodine in dichloromethane gave chiral
iodide 99 in 86% yield. The iodide was converted to triazole
(sodiumtriazole, DMF: DMPU) and immediately followed
by hydrolysis of the acetate with sodium hydroxide to provide
alcohol 100. Activation of the alcohol to the pchlorobenzene
sulfonate 101 proceeded in 76% yield which
was then coupled with commercially available amino alcohol
piperazine 102 with aqueous sodium hydroxide in DMSO to
give amine intermediate 103 in 96% yield. The amine was reacted with benzoyl chloride to give benzoate 104 (97%),
which was subsequently converted to triazine of posaconazole.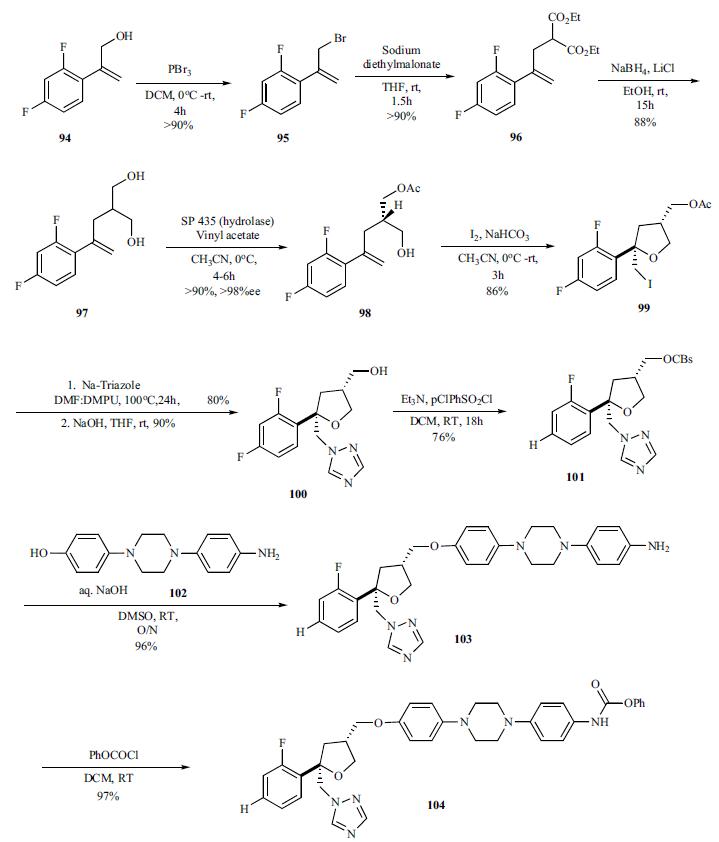
For the preparation of chiral hydrazine 107, intermediate
needed to make the triazolone, lactam 105 was reduced with
Red-Al to give (S)-2-benzyloxy propanal 106 (94%) which
was then reacted with formyl hydrazine to give hydrazone
107 in 81% yield. Addition of EtMgBr directly to formyl
hydrozones 107 gave mixture of (S,S)stereoisomer 109 and
(S,R)-diastereomer 110 in relative good diastereoselectivity
(94:6) in 55% yield. However, protection of the formyl
group as TBDMS ether 108 followed by treatment of the
EtMgCl gave 95% yield of the (S,S)-diastereomer 109 and
(S,R)-diastereomer 110 in 99:1 ratio.
For finishing off the synthesis, the formyl hydrazine 109
was coupled with the phenyl carbamate 104 in toluene at 75
- 85??C for 12 ¨C 24 hrs. After the completion of coupling, the
intermediate was heated at 100 ¨C 110??C for 24 ¨C 48 hrs to
completely cyclize to the benzyloxy triazolone 108, which
was deprotected with 5% Pd/C and formic acid at room temperature
overnight and 40??C for 24 h to give posaconazole
(XV) in 80% overall yield. 
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: concentration of fentanyl possibly
increased.
Anti-arrhythmics: avoid concomitant use with
dronedarone.
Antibacterials: rifamycins may reduce posaconazole
concentration; avoid unless benefit outweighs risk;
rifabutin concentration increased.
Anticoagulants: avoid with apixiban and rivaroxaban.
Antidepressants: avoid concomitant use with
reboxetine.
Antidiabetics: posaconazole can decrease glucose
concentrations, monitor glucose levels in diabetic
patients; possibly enhances hypoglycaemic effect of
glipizide.
Antiepileptics: phenytoin, fosphenytoin,
carbamazepine, phenobarbital and primidone may
reduce posaconazole concentration - avoid unless
benefit outweighs risk.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias with pimozide - avoid; possibly increase
quetiapine levels - reduce dose of quetiapine;
possibly increases lurasidone concentration - avoid.
Antivirals: concentration of atazanavir increased and
possibly daclatasvir and simeprevir (reduce dose of
daclatasvir, avoid with simeprevir); concentration
reduced by efavirenz and possibly fosamprenavir;
possibly increases saquinavir levels; increased
risk of ventricular arrhythmias with telaprevir;
concentration of both drugs increased with dasabuvir
and paritaprevir - avoid.
Anxiolytics and hypnotics: increases midazolam
levels.
Ciclosporin: increases posaconazole concentration;
posaconazole can increase ciclosporin concentration
- dose reduction may be required.
Cytotoxics: concentration of bosutinib increased
- avoid or reduce dose of bosutinib; possibly
increased everolimus concentration - avoid;
avoid with lapatinib; reduce dose of panobinostat
and ruxolitinib; possibly inhibits metabolism
of vinblastine and vincristine, increased risk of
neurotoxicity.
Ergot alkaloids: may increase ergot alkaloid
concentration leading to ergotism - avoid.
Guanfacine: possibly increases guanfacine
concentration - halve guanfacine dose.
Ivacaftor: possibly increased concentration of
ivacaftor.
Lipid-lowering drugs: avoid with lomitapide;
possibly increased risk of myopathy with atorvastatin
or simvastatin - avoid.1
Lumacaftor: posaconazole concentration possibly
reduced - reduce dose of lumacaftor with ivacaftor.
Ranolazine: possibly increased ranolazine
concentration - avoid.
Sirolimus: may increase concentration of sirolimus -
adjust sirolimus dose as required according to levels.
Sulphonylureas: posaconazole can decrease glucose
concentrations, monitor glucose levels in diabetic
patients.
Tacrolimus: increases Cmax and AUC of tacrolimus
by 121% and 358% respectively - reduce tacrolimus
dose to about a third of current dose and adjust as
required.
Ulcer-healing drugs: cimetidine may reduce
posaconazole concentration by 39% - avoid unless
benefit outweighs risk; avoid with histamine H2-
antagonists and proton pump inhibitors.
Metabolism
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Metabolism
Limited metabolism, most circulating metabolites are glucuronide conjugates with only small amounts of oxidative metabolites. The main elimination route of posaconazole is via the faeces (77%) where 66% of a dose is excreted unchanged. About 14% of a dose is excreted in the urine with only trace amounts excreted unchanged.
References
[1] hanan k. munayyer, paul a. mann, andrew s. chau, taisa yarosh-tomaine, jonathan r. greene, roberta s. hare, larry heimark, robert e. palermo, david loebenberg and paul m. mcnicholas. posaconazole is a potent inhibitor of sterol 14α-demethylation in yeasts and molds. antimicrobial agents and chemotherapy. 2004; 48(10): 3690-3696
[2] daryl s. schiller and horatio b. fung. posaconazole: an extended-spectrum triazole antifungal agent. clinical therapeutics. 2007; 29(9): 1862-1886
Properties of Posaconazole
| Melting point: | 170-1720C |
| Boiling point: | 850.7±75.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 14.72±0.20(Predicted) |
| color | white to beige |
| optical activity | [α]/D -24 to -32°, c = 1.0 in chloroform-d |
| Merck | 14,7602 |
| CAS DataBase Reference | 171228-49-2(CAS DataBase Reference) |
Safety information for Posaconazole
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Posaconazole
| InChIKey | RAGOYPUPXAKGKH-XAKZXMRKSA-N |
| SMILES | [C@]1(OC[C@@H](COC2C=CC(N3CCN(C4=CC=C(N5C=NN([C@@H](CC)[C@@H](O)C)C5=O)C=C4)CC3)=CC=2)C1)(CN1N=CN=C1)C1=CC=C(F)C=C1F |&1:0,3,22,25,r| |
Posaconazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
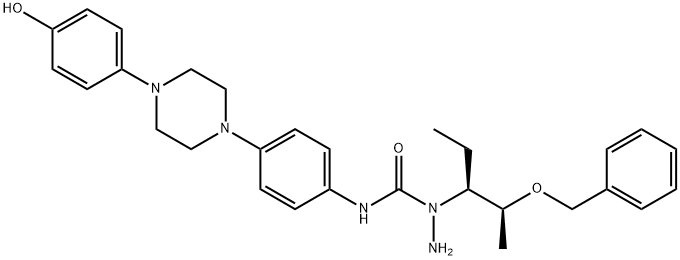
![2-[(1S,2S)-1-ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]- 3H-1,2,4-Triazol-3-one,](https://img.chemicalbook.in/CAS/GIF/184177-83-1.gif)
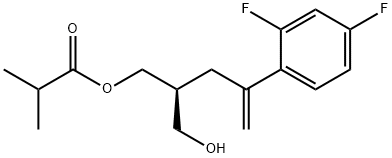
![1,3-PROPANEDIOL, 2-[2-(2,4-DIFLUOROPHENYL)-2-PROPEN-1-YL]-](https://img.chemicalbook.in/CAS/GIF/165115-73-1.gif)
![PHENYL {4-[4-(4-HYDROXYPHENYL)PIPERAZIN-1-YL]PHENYL}CARBAMATE](https://img.chemicalbook.in/CAS/GIF/184177-81-9.gif)
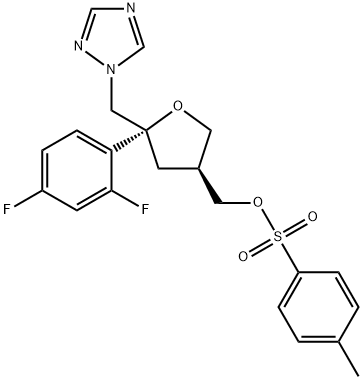
![4-Chloro-benzenesulfonic acid 5-(2,4-difluoro-phenyl)-5-[1,2,4]triazol-1-ylMethyl-tetrahydro-furan-3-ylMethyl ester](https://img.chemicalbook.in/CAS/20150408/GIF/175712-02-4.gif)
You may like
-
 171228-49-2 Posaconazole 98%View Details
171228-49-2 Posaconazole 98%View Details
171228-49-2 -
 171228-49-2 98%View Details
171228-49-2 98%View Details
171228-49-2 -
 POSACONAZOLE 17122-49-2 99%View Details
POSACONAZOLE 17122-49-2 99%View Details
17122-49-2 -
 Posaconazole 98%View Details
Posaconazole 98%View Details -
 Posaconazole 98%View Details
Posaconazole 98%View Details -
 Posaconazole 98% (HPLC) CAS 171228-49-2View Details
Posaconazole 98% (HPLC) CAS 171228-49-2View Details
171228-49-2 -
 Posaconazole Api PowderView Details
Posaconazole Api PowderView Details
171228-49-2 -
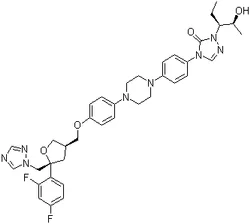 PosaconazoleView Details
PosaconazoleView Details
171228-49-2
