Iron(III) fluoride
Synonym(s):Ferric fluoride
- CAS NO.:7783-50-8
- Empirical Formula: F3Fe
- Molecular Weight: 112.84
- MDL number: MFCD00016093
- EINECS: 232-002-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
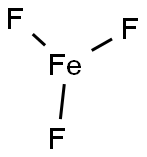
What is Iron(III) fluoride?
Chemical properties
green powder
Chemical properties
Iron(III) fluoride is only sparingly soluble in water and insoluble in alcohol and ether. When heated in hydrogen it is reduced to iron(II) fluoride and thence to the metal. In aqueous solution the hydrolysis appears to be very slight ; in the presence of alkali metal fluorides, complex fluorides of types MFeF4, M2FeF5.xH2O and M3FeF6 can be crystallized. The magnetic behaviour of FeF3 is complicated; between 63° and 293°K the susceptibility is field strength dependent. The trihydrate has a magnetic moment μ = 2-25 B.M. at 300°K.
The Uses of Iron(III) fluoride
As catalyst in organic reactions.
The Uses of Iron(III) fluoride
Iron(III) fluroide is used in ceramics manufacturing. It is used as a catalyst for certain cross coupling reactions. Further, it is involved in the chemoselective addition of cyanide to aldehyde to prepare cyanohydrins.
Preparation
This can be prepared by the action of fluorine on the metal,
iron(II), or iron(III) chloride or by dehydration of the hydrate (obtained from aqueous
solution) by heating in a current of hydrogen fluoride. A convenient laboratory preparation involves the reaction of hydrogen fluoride with anhydrous iron(III) chloride
at room temperature :
FeCl3+3HF→ FeF3+3HCl
When crystallized from aqueous solution (iron(III) "hydroxide" and aqueous hydrofluoric acid) it forms the pale pink 4?-hydrate (room temperature evaporation) and the pink 3-hydrate (evaporation by heating).
General Description
IRON (II) FLUORIDE is a green crystalline solid. IRON (II) FLUORIDE is slightly soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. IRON (II) FLUORIDE is used in ceramics.
Air & Water Reactions
Slightly soluble in water forming acidic solutions.
Reactivity Profile
Acidic salts, such as IRON (II) FLUORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. IRON (II) FLUORIDE is used to catalyze organic reactions.
Hazard
Strong irritant.
Health Hazard
INHALATION: Inorganic fluorides are generally irritating. INGESTION: Ingestion of iron compounds can cause: lethargy, retching, vomiting, tarry stools, fast and weak pulse, low blood pressure, and coma.
Fire Hazard
Behavior in Fire: May give off fumes or vapors of fluorides; hydrofluoric acid.
Safety Profile
Moderately toxic by intraperitoneal route. Mutation data reported. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES.
Properties of Iron(III) fluoride
| Melting point: | 1000 °C |
| Density | 3,87 g/cm3 |
| solubility | insoluble in ethanol, ethyl ether, benzene |
| form | Powder |
| color | Pale green |
| Specific Gravity | 3.87 |
| Water Solubility | Soluble in dilute hydrofluroic acid. Slightly soluble in water. Insoluble in alcohol, ether and benzene. |
| Sensitive | Hygroscopic |
| Merck | 14,4022 |
| Solubility Product Constant (Ksp) | pKsp: 5.63 |
| Exposure limits | ACGIH: TWA 1 mg/m3; TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 1 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7783-50-8(CAS DataBase Reference) |
| EPA Substance Registry System | Ferric fluoride (7783-50-8) |
Safety information for Iron(III) fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Iron(III) fluoride
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Iron(III) fluoride CAS 7783-50-8View Details
Iron(III) fluoride CAS 7783-50-8View Details
7783-50-8 -
 Iron(III) fluoride CAS 7783-50-8View Details
Iron(III) fluoride CAS 7783-50-8View Details
7783-50-8 -
 Iron(III) fluoride CAS 7783-50-8View Details
Iron(III) fluoride CAS 7783-50-8View Details
7783-50-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1