ANTIMONY PENTAFLUORIDE
Synonym(s):Antimony pentafluoride;Antimony(V) fluoride
- CAS NO.:7783-70-2
- Empirical Formula: F5Sb
- Molecular Weight: 216.75
- MDL number: MFCD00011219
- EINECS: 232-021-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
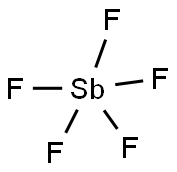
What is ANTIMONY PENTAFLUORIDE?
Description
This week’s molecules combine to make “magic”. The first, fluorosulfuric acid (HSO3F; also called fluorosulfonic acid) is an extremely strong Br?nsted acid that has been known since the late 19th century. It is ≈1000 times stronger than pure sulfuric acid, which puts it in the class of superacids. It can protonate and dissolve almost all organic compounds and has several industrial uses, including catalysis and hydrofluorination.
The second molecule, antimony pentafluoride (SbF5), was first reported in 1904. This strong Lewis acid is nasty: It reacts violently with water, attacks the skin and eyes, and corrodes metals. Its chief use is the fluorination of organic compounds.
So where’s the magic? In the 1960s, George A. Olah and co-workers, first at Dow Chemical (Midland, MI), then at Case Western Reserve University (Cleveland), were seeking ways to make and study stable carbocations. To extract hydride groups (hydrogen anions) from organic compounds, they needed a strong, but poorly nucleophilic, acid.
At Case Western, the researchers discovered that a mixture of HSO3F and SbF5easily dissolves paraffins. NMR spectra of the solutions showed that the acidic combination cleaved the long-chain alkanes, and the fragments isomerized to form stable?tert-butyl cations (Me3C+). This result was so spectacular that the chemists dubbed the mixture “magic acid”. The usual molar ratio of the components is 1:1, but other combinations have also been useful.
Olah went on to do extensive research on carbocations at Case Western and then the University of Southern California (Los Angeles). He received the Nobel Prize in Chemistry in 1994 for his pioneering work.
Description
Antimony pentafluoride is a noncombustible,oily, colorless liquid with a pungent odor. Molecularweight= 216.75; Boiling point= 143℃; Melting/Freezingpoint=8.3℃; Specific gravity: 2.340 at 30℃; Liquidsurface tension= 5 (estimate) 0.020 N/m at 20℃; Latentheat of vaporization= (estimate) 1.8 3 105 J/kg; Vaporpressure= 1.33 kPa at 25℃. Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 0,Reactivity 1. Soluble in water.
Chemical properties
colourless oily liquid
Chemical properties
Antimony pentafluoride is a noncombustible, oily, colorless liquid with a pungent odor.
Physical properties
Colorless oily liquid; highly viscous; hygroscopic; freezes at 8.3°C; boils at 149.5°C; density 2.99 g/cm3 at 23°C; soluble in excess water (with violent reaction) and glacial acetic acid; also soluble in potassium fluoride.
The Uses of ANTIMONY PENTAFLUORIDE
HF/SbF5 is an excellent medium for the preparation of alkylidene oxonium salts.1
The Uses of ANTIMONY PENTAFLUORIDE
In the fluorination of organic Compounds, see the monograph Preparation, Properties and Technology of Fluorine and Organic Fluoro Compounds, C. Slesser, S. R. Schram, Eds. (McGraw-Hill, New York, 1951) 868 pp.
Preparation
Antimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:
SbCl5 +5 HF → SbF5 +5 HCl
It may also be prepared from antimony trifluoride and fluorine, or by treating antimony pentaoxide with aqueous hydrofluoric acid and evaporing water.
Definition
ChEBI: Antimony pentafluoride is an antimony molecular entity and an inorganic fluoride salt. It has a role as a Lewis acid.
Production Methods
Antimony pentafluoride is prepared by treating antimony pentachloride with excess anhydrous hydrogen fluoride. The antimony pentafluoride is separated from the volatile byproducts by fractional distillation. It can also be prepared by allowing fluorine to react with antimony powder or molten antimony trifluoride. The main use of antimony pentafluoride is as a fluorinating agent.
General Description
A colorless, oily liquid. Fumes irritate the eyes and mucous membranes. Toxic. Corrosive to metals and tissue. Extremely dangerous to tissue; its burns may be followed by gangrene. Only shipped in cylinders. Under prolonged exposure to heat cylinders may violently rupture and rocket. Used to make other chemicals as well as a catalyst in the manufacture of other chemicals.
Reactivity Profile
ANTIMONY PENTAFLUORIDE is strongly acidic. Reacts vigorously with bases. When heated to decomposition, ANTIMONY PENTAFLUORIDE emits highly toxic fumes of fluorides and metallic antimony. Reacts with ammonia to form a diammoniate.
Hazard
Corrosive to skin and tissue.
Health Hazard
The compound is irritating to eyes, skin, and lungs. Contact with eyes or skin causes severe burns. The compound is extremely toxic with a probable oral lethal dose of 5-50 mg/kg or between 7 drops and one teaspoonful for a 150 pound person (antimony salts).
Fire Hazard
Reacts violently with water, to form poisonous hydrogen fluoride fumes. If confined and wet can cause explosion. May cause fire in contact with combustible material. Hazardous polymerization may not occur.
Safety Profile
A poison by inhalation. A very reactive, corrosive liquid to skin, eyes, mucous membranes. See also FLUORIDES and ANTIMONY COMPOUNDS. Violent reaction with phosphates. When heated to decomposition it emits very toxic fumes of F and Sb.
Potential Exposure
It is used as a catalyst in chemical reactions or as a source of fluorine (fluorinating reagent) in fluorination reactions.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24°48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Antimony pentafluoride must be stored to avoid contactwith phosphorus, phosphates, siliceous, and combustible ororganic materials since violent reactions occur. Store intightly closed containers in a cool, well-ventilated area awayfrom water or moisture and heat. Outside or detached storage is preferred.
Shipping
UN1732 Antimony pentafluoride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materials.
Purification Methods
Purify it by vacuum distillation, preferably in a quartz apparatus, and store it in quartz or aluminum bottles. It is a hygroscopic viscous liquid which reacts violently with H2O and is hydrolysed by alkalis. It is POISONOUS and attacks the skin. [Woolf & Greenwood J Chem Soc 2200 1950, Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 200 1965.]
Incompatibilities
Water and other forms of moisture releases hydrofluoric acid, combustible organic and siliceous materials, phosphorus, and phosphate materials. Attacks glass, ceramic, lead, and metals, including copper in the presence of moisture. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Antimony pentafluoride, if confined and wet may cause explosion. Antimony pentafluoride appears to have oxidizing properties; may cause fire in contact with combustible or organic materials. May react with metals, including lead, releasing flammable hydrogen gas.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of ANTIMONY PENTAFLUORIDE
| Melting point: | 7 °C(lit.) |
| Boiling point: | 149.4 °C |
| Density | 2.993 g/mL at 25 °C(lit.) |
| vapor density | 2.2 (vs air) |
| vapor pressure | 10 mm Hg ( 25 °C) |
| Flash point: | 149.5°C |
| storage temp. | Store below +30°C. |
| solubility | liquid sulfur dioxide: slightly soluble(lit.) |
| form | Shiny Flakes or Powder |
| color | White to off-white |
| Water Solubility | reacts vigorously with H2O, becoming hydrolyzed [HAW93] [KIR78] |
| Sensitive | Moisture Sensitive |
| Merck | 13,702 |
| CAS DataBase Reference | 7783-70-2(CAS DataBase Reference) |
| EPA Substance Registry System | Antimony pentafluoride (7783-70-2) |
Safety information for ANTIMONY PENTAFLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ANTIMONY PENTAFLUORIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

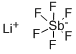
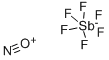
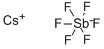

You may like
-
 Antimony pentafluoride, 99.5+% CAS 7783-70-2View Details
Antimony pentafluoride, 99.5+% CAS 7783-70-2View Details
7783-70-2 -
 Antimony(V) fluoride CAS 7783-70-2View Details
Antimony(V) fluoride CAS 7783-70-2View Details
7783-70-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
