Aluminum fluoride
Synonym(s):Aluminum trifluoride;Trifluoroaluminum
- CAS NO.:7784-18-1
- Empirical Formula: AlF3
- Molecular Weight: 83.98
- MDL number: MFCD00003426
- EINECS: 232-051-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:02
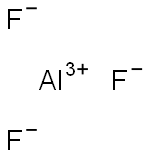
What is Aluminum fluoride?
Chemical properties
Aluminum fluoride, AlF3, is an anhydrous crystalline powder with a melting point of 1291 "C. Aluminum fluoride (hydrated), AlF3·31/2H20, is a white crystalline powder that is insoluble in water.
The Uses of Aluminum fluoride
Aluminum fluoride is used to produce lowmelting aluminum metal, as a flux in ceramic glazes and white enamels, and as a catalyst in chemical reactions.
The Uses of Aluminum fluoride
Production of aluminum to lower the melting point and increase the conductivity of the electrolyte, flux in ceramic glazes and enamels, manufacture of aluminum silicate, catalyst.
The Uses of Aluminum fluoride
In ceramics, as flux in metallurgy, in aluminum manufacture, as inhibitor of fermentation, as catalyst in organic reactionsAluminum fluoride is used in the smelting process to lower the melting point of electrolytes, production of aluminum silicates, refining of aluminum scarp, production of specialty refractory products, and in glass industry as filler. It is used as flux ingredient to remove magnesium.
General Description
Odorless white powder or granules. Denser than water. Solubility in water at 25°C equals 0.559 g / 100 mL.
Air & Water Reactions
Slightly soluble in water
Reactivity Profile
Aluminum fluoride when heated to sublimation condition, emits toxic fumes of fluoride [USCG, 1999].
Hazard
Strong irritant to tissue.
Health Hazard
ACUTE: respiratory irritation; possible nose bleeding or vomiting; CHRONIC: aggravates bronchitis/asthma; increased bone density.
Fire Hazard
Special Hazards of Combustion Products: When heated to sublimation condition, emits toxic fumes of fluoride
Flammability and Explosibility
Non flammable
Safety Profile
A poison by ingestion. Moderately toxic by subcutaneous route. A severe eye irritant. Violently impact-sensitive when in contact with Na and K. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and ALUMINUM COMPOUNDS.
Potential Exposure
Used as component of electrolyte from which aluminum metal is produced; in the manufacture of ceramics, enamels, aluminum silicate; as flux in metallurgy; as a fermentation inhibitor
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Reacts violently with potassium or sodium
Waste Disposal
Neutralize with soda ash; add slaked lime; let stand for 24 hours. Transfer sludge to sewage facility.
Properties of Aluminum fluoride
| Melting point: | 1290 °C (lit.) |
| Boiling point: | 1291 °C |
| Density | 3.1 g/mL at 25 °C (lit.) |
| Flash point: | 1250°C |
| solubility | Sparingly soluble in acids and alkalies. Insoluble in Acetone. |
| form | powder |
| color | White to light gray |
| Specific Gravity | 2.882 |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,339 |
| Sublimation | 1250 ºC |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2 mg/m3; TWA 2.5 mg/m3 |
| Dielectric constant | 2.2(Ambient) |
| CAS DataBase Reference | 7784-18-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Aluminum trifluoride(7784-18-1) |
| EPA Substance Registry System | Aluminum trifluoride (7784-18-1) |
Safety information for Aluminum fluoride
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Aluminum fluoride
Aluminum fluoride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
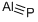


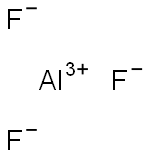
You may like
-
 Aluminium Fluoride 99%View Details
Aluminium Fluoride 99%View Details -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
Aluminum fluoride, Anhydrous CAS 7784-18-1View Details
7784-18-1 -
 NRE Technical Grade Aluminum Fluoride PowderView Details
NRE Technical Grade Aluminum Fluoride PowderView Details
13775-53-6
