Magnesium fluoride
Synonym(s):MG516100;Sellaite
- CAS NO.:7783-40-6
- Empirical Formula: F2Mg
- Molecular Weight: 62.3
- MDL number: MFCD00011108
- EINECS: 231-995-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
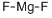
What is Magnesium fluoride?
Description
Magnesium fluoride (MgF2) is a white crystalline salt. It is used in the electrolysis of aluminum ore to produce metallic aluminum and also as a reflective coating on various types of optical components. It is a tetragonal, birefringent crystal with the TiO2 type of structure.
Chemical properties
white to light beige powder
Chemical properties
Magnesium Fluoride is a fine white crystalline powder with low chemical reactivity. This relative inertness makes possible some of its uses, eg, stable permanent films to alter light transmission properties of optical and electronic materials.
The Uses of Magnesium fluoride
Suitable for vacuum deposition. Magnesium fluoride occurs in nature as the mineral, sellaite. It is used in
glass and ceramics. Single crystals are used for polarizing prisms and lenses.
The Uses of Magnesium fluoride
In the ceramics and glass industry.Magnesium Fluoride is a durable crystal with low absorption, suitable for high-powered laser, space, and other UV applications. MgF2 is also naturally birefringent, making it an ideal material for use where this property can be exploited, such as retardation plates and polarizing elements, particularly in the wavelength range from 0.13-0.30 µm.
The Uses of Magnesium fluoride
Magnesium fluoride (MgF2) is used to polarize corrective lenses of eyeglasses to reduce the glare of sunlight by selecting the orientation of the light waves passing through the lenses. MgF2 is also used to polarize windows, sunglasses, and similar optical items.
Preparation
Magnesium fluoride is a colorless salt with the rutile structure. It is formed by reaction of magnesium oxide and HF or magnesium carbonate and NH4F·HF. It is also a by product from the manufacture of elements such as beryllium by reduction of the corresponding fluoride by magnesium metal.
Hazard
Strong irritant. TLV: 2.5 mg(F)/m3.
Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of F-. See also MAGNESIUM and FLUORIDES.
Properties of Magnesium fluoride
| Melting point: | 1263 °C (lit.) |
| Boiling point: | 2227 °C/1 atm (lit.) |
| Density | 3.15 g/mL at 25 °C (lit.) |
| refractive index | 1.365 |
| form | random crystals |
| color | White to off-white |
| Specific Gravity | 3.15 |
| Water Solubility | 87 mg/L (18 ºC) |
| Crystal Structure | Tetragonal, Rutile |
| Merck | 14,5665 |
| Solubility Product Constant (Ksp) | pKsp: 10.29 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| InChI | InChI=1S/2FH.Mg/h2*1H;/q;;+2/p-2 |
| CAS DataBase Reference | 7783-40-6(CAS DataBase Reference) |
| EPA Substance Registry System | Magnesium fluoride (7783-40-6) |
Safety information for Magnesium fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Magnesium fluoride
| InChIKey | ORUIBWPALBXDOA-UHFFFAOYSA-L |
| SMILES | [Mg](F)F |
Magnesium fluoride manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
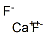
You may like
-
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6 -
 Magnesium fluoride CAS 7783-40-6View Details
Magnesium fluoride CAS 7783-40-6View Details
7783-40-6
