Nickel fluoride
Synonym(s):Nickel difluoride;Nickel fluoride;Nickelous fluoride
- CAS NO.:10028-18-9
- Empirical Formula: F2Ni
- Molecular Weight: 96.69
- MDL number: MFCD00016263
- EINECS: 233-071-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-12 17:37:50
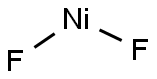
What is Nickel fluoride?
Chemical properties
Green crystalline powder
Chemical properties
Nickel(II) fluoride has the tetragonal rutile structure with only slightly tetragonally compressed NiF6 octahedra; the Ni-F distances being 2.01 and 1.98 ?. Above the melting point it sublimes as a linear gaseous molecule. The anhydrous fluoride is rather unreactive towards concentrated acids; it is only slightly soluble in anhydrous hydrogen fluoride and is not attacked by electronegative elements such as chlorine, sulphur and phosphorus. The pale green tetrahydrate is slightly soluble in water; when heated, several intermediate hydrates are formed prior to N1F2.
The Uses of Nickel fluoride
Nickel fluoride?comprises the passivating surface that forms on nickel alloys, e.g. monel, which is why such materials are good to store or transport hydrogen fluoride or elemental fluorine.
Preparation
Nickel fluoride tetrahydrate [13940-83-5], NiF2.4H2O, and its anhydrous counterpart, nickel fluoride [10028-18-9], NiF2 , are the only known stable binary compounds of nickel and fluorine. The former is a greenish light yellow crystal or powder prepared by the addition of nickel carbonate to 30-50% aqueous HF solution. The nickel fluoride formed first goes into solution and then precipitates out as the tetrahydrate as the concentration of Nickel fluoride increases and that of HF decreases. When the addition of nickel is complete, the solution and the precipitates are dried at 75-100°C until all the water is expelled. The tetrahydrate has high solubility in aqueous HF, eg, 13.3 wt % in 30% HF. It is slightly soluble in water and insoluble in alcohol and ether.
Anhydrous Nickel fluoride, a light yellow colored powder, is prepared by the action of anhydrous HF on anhydrous NiCl2 , or nickel fluoride tetrahydrate at 300°C. It is also prepared by heating a mixture of NH4.HF2 and NiF2.4H2O. The other methods include the fluorination of metal salts using excess SF4 or using ClF3 at elevated temperatures, or the reaction of NiCO3 and anhydrous HF at 250°C.
Flammability and Explosibility
Non flammable
Safety Profile
NTP 10th Report on Carcinogens. Reacts violently with potassium. Chronic exposure may cause mottling of teeth, changes in bones. Mutation data reported. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and NICKEL COMPOUNDS.
Properties of Nickel fluoride
| Melting point: | 1380℃(lit.) |
| Boiling point: | 1498.77°C (estimate) |
| Density | 4.72 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| solubility | diethyl ether: insoluble(lit.) |
| pka | 0.019[at 20 ℃] |
| form | Crystalline |
| Specific Gravity | 4.72 |
| color | Pale yellow |
| Water Solubility | 2.51 g/100 mL |
| Sensitive | Hygroscopic |
| Merck | 14,6508 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3; TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; IDLH 250 mg/m3; TWA 2.5 mg/m3; TWA 0.015 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 10028-18-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Nickel difluoride(10028-18-9) |
| EPA Substance Registry System | Nickel fluoride (NiF2) (10028-18-9) |
Safety information for Nickel fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nickel fluoride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 10028-18-9 NICKEL FLUORIDE 98%View Details
10028-18-9 NICKEL FLUORIDE 98%View Details
10028-18-9 -
 Nickel(II) fluoride 98%View Details
Nickel(II) fluoride 98%View Details -
 Nickel(II) fluoride 10028-18-9 98%View Details
Nickel(II) fluoride 10028-18-9 98%View Details
10028-18-9 -
 10028-18-9 Nickel(II) fluoride 99%View Details
10028-18-9 Nickel(II) fluoride 99%View Details
10028-18-9 -
 Nickel(II) fluoride, Anhydrous CAS 10028-18-9View Details
Nickel(II) fluoride, Anhydrous CAS 10028-18-9View Details
10028-18-9 -
 Nickel(II) fluoride, Anhydrous CAS 10028-18-9View Details
Nickel(II) fluoride, Anhydrous CAS 10028-18-9View Details
10028-18-9 -
 Nickel fluoride CAS 10028-18-9View Details
Nickel fluoride CAS 10028-18-9View Details
10028-18-9 -
 Nickel fluoride 95% CAS 10028-18-9View Details
Nickel fluoride 95% CAS 10028-18-9View Details
10028-18-9
