Fesoterodinefumarate
- CAS NO.:286930-03-8
- Empirical Formula: C30H41NO7
- Molecular Weight: 527.65
- MDL number: MFCD12756004
- EINECS: 639-689-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
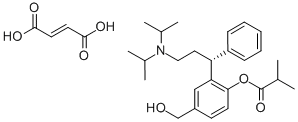
What is Fesoterodinefumarate?
Chemical properties
White Solid
The Uses of Fesoterodinefumarate
Fesoterodine fumarate (Toviaz) is an antimuscarinic agent and is rapidly de-esterified to its active metabolite 5-hydroxymethyl tolterodine that is a muscarinic receptor antagonist. Fesoterodine fumarate (Toviaz) is used to treat the symptoms of overactiv
The Uses of Fesoterodinefumarate
(R)-Fesoterodine Fumarate is a muscarinic receptor antagonist for the treatment of Lower Urininary Tract Symptoms (LUTS). It is very similar to Tolterodine (T535800).
What are the applications of Application
(R)-Fesoterodine Fumarate is a mAChR M antagonist that blocks acetylcholine binding
Clinical Use
Antimuscarinic:Symptomatic treatment of urinary incontinence, frequency or urgency
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased risk of antimuscarinic
side effects with disopyramide.
Antifungals: dose reduction advised with
itraconazole and ketoconazole.
Antivirals: dose reduction advised with atazanavir,
indinavir, ritonavir and saquinavir.
Induction of CYP3A4 may lead to subtherapeutic
plasma levels. Concomitant use with CYP3A4
inducers (e.g. carbamazepine, rifampicin,
phenobarbital, phenytoin, St John's Wort) is not
recommended.
Co-administration of a potent CYP2D6 inhibitor
may result in increased exposure and adverse events.
A dose reduction to 4 mg may be needed'
Metabolism
Rapidly and extensively hydrolysed to its active metabolite. The active metabolite is further metabolised in the liver to its carboxy, carboxy-N-desisopropyl, and N-desisopropyl metabolites via two major pathways involving CYP2D6 and CYP3A4. None of these metabolites contribute significantly to the antimuscarinic activity of fesoterodine. Approximately 70% of an oral dose of fesoterodine is recovered in the urine as metabolites, and a smaller amount in the faeces.
Properties of Fesoterodinefumarate
| Melting point: | 72-78°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Fesoterodinefumarate
Computed Descriptors for Fesoterodinefumarate
Fesoterodinefumarate manufacturer
Lupin Ltd
Ralington Pharma
BDR Pharmaceuticals International Pvt Ltd
Raks Pharma Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 286930-03-8 Fesoterodine fumarate 99%View Details
286930-03-8 Fesoterodine fumarate 99%View Details
286930-03-8 -
 286930-03-8 98%View Details
286930-03-8 98%View Details
286930-03-8 -
 Fesoterodine fumarate 98%View Details
Fesoterodine fumarate 98%View Details
286930-03-8 -
 Fesoterodine fumarate 286930-03-8 98%View Details
Fesoterodine fumarate 286930-03-8 98%View Details
286930-03-8 -
 Fesoterodine fumarate 99%View Details
Fesoterodine fumarate 99%View Details
286930-03-8 -
 Fesoterodine fumarate 98% (HPLC) CAS 286930-03-8View Details
Fesoterodine fumarate 98% (HPLC) CAS 286930-03-8View Details
286930-03-8 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1