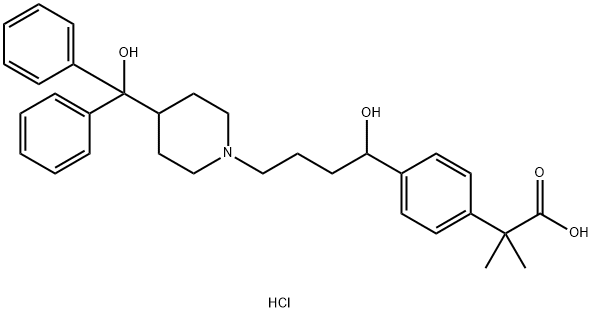
Fexofenadine hydrochloride
Synonym(s):Fexofenidine hydrochloride;MDL 16455 hydrochloride;Terfenidine carboxylate hydrochloride
- CAS NO.:153439-40-8
- Empirical Formula: C32H40ClNO4
- Molecular Weight: 538.13
- MDL number: MFCD08064193
- EINECS: 604-906-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
What is Fexofenadine hydrochloride ?
Description
Fexofenadine hydrochloride (FEX) is obtained as a white to off-white crystalline powder. FEX is a nonsedating, second-generation H1 antihistamine which acts as a reversible and competitive inhibitor at H1 histamine receptor sites. The drug is an active carboxylic acid metabolite of terfenadine and provides all the therapeutic benefits of terfenadine while avoiding the serious cardiotoxic and drug interaction risks of the parent drug and is, therefore, considered a relatively safe alternative to terfenadine. The synthetic approach for preparation of FEX involves the reduction of a carboxylate derivative, 4-[4-[4-(hydroxybiphenylmethyl)-1-piperidinyl]-1-oxobutyl]-a,a-dimethyl benzene acetate; followed by hydrolysis with a base, for example, alkali metal hydroxides, to get the carboxylic acid derivative FEX. FEX exists as a zwitterion in aqueous media at physiological pH. The drug has two ionization groups corresponding to the free carboxylic group on the side chain and the substituted ring nitrogen, contributing to a pKa value of 4.25 and 9.53, respectively. FEX is stable under normal storage conditions, but the substance is stored in tight containers and protected from light and humidity. Photodegradation of FEX is studied using a stability indicating high performance liquid chromatography (HPLC) assay method.
Chemical properties
Off-White Crystalline Solid
Originator
Alernex,Dabur Pharmaceuticals Ltd.,India
The Uses of Fexofenadine hydrochloride
Fexofenadine hydrochloride is a non-sedating antihistamine that inhibits Cox-1, Cox-2, and is a histamine H1 receptor agonist and exhibits anti-inflammatory effects. It is devoid of central nervous system effects in part because it is a good substrate for the P-glycoprotein efflux pump situated within the blood-brain barrier. It is a metabolite of of terfenadine, a H1-Histamine receptor antagonist.
Definition
ChEBI: Fexofenadine hydrochloride is a diarylmethane.
Manufacturing Process
Microbiological Preparation of Fexofenadine (Patent U.S. 6,558,931)
Ten 250 ml Erlenmeyer flasks containing 100 ml of medium D are seeded with
A. corymbifera LCP 63-1800. 50 mg of Terfenadine in 1 ml of ethanol is added
to each Erlenmeyer flask. The content of 10 Erlenmeyer flasks are filtered on
gauze (the supernatant has a pH equal to 8.0), and saturation with sodium
chloride is carried out for 2 hours (pH = 5-6), followed by extracting 3 times
with ethyl acetate and drying over magnesium sulfate. After evaporation
under reduced pressure, 409 mg of expected crude product is obtained (yield
= 77%, approximately 90% pure product). This product is purified on a
column of silica gel (230-400 mesh, 40 g of silica, diameter = 3 cm) eluting
with a methylene chloride/methanol/ammonium hydroxide mixture
(82.5:15:2.5). 312 mg of pure Fexofenadine is recovered (61.4%).
Synthesis of Fexofenadine (Patent U.S. No. 5,578,610
Aluminum chloride (44 g; 0.33 mol) was added in portions to a solution of
freshly distilled 4-chlorobutyryl chloride (17 mL; 0.15 mol) in 460 mL of
carbon disulfide at -10°C under a nitrogen atmosphere. The mixture was
stirred for 15 min, then the cooling bath was removed and the mixture was
allowed to warm to ambient temperature. The mixture was stirred then for 15
min more, then cooled again to -10°C and a solution of ethyl-α,α-
dimethylphenyl acetate (26.6 g; 0.14 mol) in 70 mL of carbon disulfide was
added dropwise. The mixture was stirred for 3 hours, then stirred overnight at
room temperature. The reaction mixture was partitioned between water and
CHCl3. The combined organic portions were dried over MgSO4, filtered and
concentrated in vacuo. The residue was dissolved in CH2Cl2 and filtered
through a plug of SiO2, eluting with 10% EtOAc in hexane. Yield of ethyl 3-
and 4-(4-chloro-1-oxobutyl)-α,α-dimethylphenylacetate 39.4 g (as a mixture
of aromatic regioisomers).
To a solution of 39.4 g of ethyl 3- and 4-(4-chloro-1-oxobutyl)-α,α-
dimethylphenylacetate dissolved in 800 mL of methanol and 200 mL of water
was added 40 g of NaOH. The mixture was refluxed for one hour. The cooled
mixture was then concentrated in vacuo. The concentrate was diluted with
water and washed with 2 portions of EtOAc. The aqueous layer was acidified
with concentrated HCl and extracted with 2 portions of EtOAc. The extracts
were dried over MgSO4, filtered, and concentrated in vacuo to afford 30.3 g of
crude product. The crude product was dissolved in 600 mL of EtOAc, 38 g of
cinchonidine was added, and the mixture was stirred overnight. The resulting
solids were filtered and washed with EtOAc and sucked dry under a rubber
dam to afford 25 g of a solid 4-(cyclopropyl-oxo-methyl)-α,α-
dimethylphenylacetic acid.
A solution of 10.5 g of 4-(cyclopropyl-oxo-methyl)-α,α-dimethylphenylacetic
acid in 250 mL of CH2Cl2 was cooled in an ice-MeOH bath and 25 g of
trimethylsilyliodide was then added via pipette. The mixture was stirred in the
ice bath for one hour, warmed to ambient temperature, and stirred for one
hour. A solution of aqueous sodium bisulfite was then added and the mixture
was stirred well. The phases were partitioned and the aqueous layer was
extracted with CH2Cl2. The combined organics were washed with saturated
aqueous NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford
12.6 g (77%) of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid.
To a solution of 12.6 g of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid
in 100 mL of ether cooled in an ice bath, was added 40 mL of ethereal CH2N2.
The mixture was stirred at 0°C for few minutes, then let stand for 2 hours. A
few drops of AcOH were added to decompose excess CH2N2, then the mixture
was filtered and stripped to afford 12.6 g (96%) of methyl 4-(4-iodo-1-
oxobutyl)-α,α-dimethylphenylacetate.
A solution of 12.6 g of methyl 4-(4-iodo-1-oxobutyl)-α,α-
dimethylphenylacetate in 500 mL of toluene in a one liter three neck flask was
added 8.8 g of 4-(α,α-diphenyl)piperidinemethanol and 23 g of K2CO3 and the
mixture was refluxed for 7 hours. The cooled reaction mixture was then
filtered and concentrated in vacuo. The residue was dissolved in ether and
treated with excess ethereal HCl. The mixture was then concentrated to a
solid. The solid was treated with EtOAc and collected by filtration. The product
was then partitioned between EtOAc and 2 N Na2CO3. The organics were dried
over MgSO4, filtered, and concentrated in vacuo to afford 13.5 g (79%) of
methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-oxobutyl]-α,α-
dimethylphenylacetate.
A solution of 13.5 g of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-
1-oxobutyl]-α,α-dimethylphenylacetate in 250 mL of methanol was cooled in
an ice-methanol bath and 1.8 g of NaBH4 was added in portions. After 1 hour,
the mixture was concentrated to a solid. The residue was partitioned between
EtOAc and saturated aqueous NaHCO3. The aqueous portion was extracted
with EtOAc. The combined organics were washed with saturated aqueous
NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford 9.5 g
(70%) of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-
hydroxybutyl]-α,α-dimethylphenylacetate as a foam.
To a solution of 9.5 g of methyl-4-[4-[4-(hydroxydiphenylmethyl)-1-
piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetate in 300 mL of methanol
and 150 mL of water was added 10 g of NaOH. The mixture was refluxed for 1
hour, then cooled. The methanol was removed in vacuo. The concentrate was
diluted with water and CHCl3 and the pH adjusted to approximately 5.5 to 6.0.
The phases were separated and the aqueous phase was extracted with CHCl3.
The combined organics were dried over MgSO4, filtered, and stripped to afford
9.0 g of crude product. The crude product was dissolved in CH2Cl2 and
chromatographed on Davisil Grade 633 SiO2 eluting with a gradient of CHCl3,
to 10% of methanol in CHCl3, to 25% of methanol in CHCl3. The product was
concentrated to afford 5.2 g of white crystals of 4-[4-[4-
(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetic acid (Fexofenadine).
In practice it is usually used as hydrochloride salt.
Therapeutic Function
Antihistaminic
General Description
Fexofenadine hydrochloride, (±)-4-[1-hydroxy-4-[4-(hydroxyldiphenylmethyl)- 1-piperinyl]butyl-α,α-dimethylbenzeneacetic acid (Allegra), occurs as a white to off-white crystalline powder that is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane.
This compound is marketed as a racemate and is a zwitterion in aqueous media at physiological pH. Fexofenadine is a primary oxidative metabolite of terfenadine. Terfenadine was developed during a search for new butyrophenone antipsychotic drugs, as evidenced by the presence of the N-phenylbutanol substituent. It also contains a diphenylmethyl-piperidine moiety analogous to the piperazine antihistamines.
Biological Activity
Fexofenadine hydrochloride is a selective histamine H1 receptor antagonist (pKi = 8.1). Active metabolite of Terfenadine that displays non-sedating antiallergic effects. Fexofenadine hydrochloride counteracts histamine mediated immune suppression and improves immunotherapy response.
Biochem/physiol Actions
Fexofenadine is a non-sedating H1 histamine receptor antagonist.
Clinical Use
Antihistamine:Symptomatic relief of rhinitis and urticaria
Drug interactions
Potentially hazardous interactions with other drugs Antibacterials: effects possibly reduced by rifampicin. Antivirals: concentration possibly increased by ritonavir. Aluminium/magnesium containing antacids: reduced absorption - avoid for 2 hours.
Metabolism
Fexofenadine undergoes negligible metabolism (hepatic or non-hepatic); about 5% of the total dose is metabolised, mostly by the intestinal mucosa, with 0.5-1.5% of the dose undergoing hepatic biotransformation by the cytochrome P450 system. The major route of elimination is believed to be via biliary excretion while up to 10% of ingested dose is excreted unchanged through the urine.
Properties of Fexofenadine hydrochloride
| Melting point: | 148-150oC |
| RTECS | CY1633377 |
| storage temp. | 2-8°C |
| solubility | Soluble in Dimethyl sulfoxide (50 mM), and methanol. |
| form | powder |
| color | white to beige |
| Merck | 14,4068 |
| CAS DataBase Reference | 153439-40-8(CAS DataBase Reference) |
Safety information for Fexofenadine hydrochloride
Computed Descriptors for Fexofenadine hydrochloride
| InChIKey | RRJFVPUCXDGFJB-UHFFFAOYSA-N |
Fexofenadine hydrochloride manufacturer
Global Chemie
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Pharma Links
Festiva Pharma
Bazayan & Co.
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Fexofenadine Hydrochloride 99%View Details
Fexofenadine Hydrochloride 99%View Details -
 Fexofenadine Hydrochloride IP/BP/USP/EP 99%View Details
Fexofenadine Hydrochloride IP/BP/USP/EP 99%View Details
153439-40-8 -
 Fexofenadine hydrochloride 98%View Details
Fexofenadine hydrochloride 98%View Details -
 153439-40-8 95-99 %View Details
153439-40-8 95-99 %View Details
153439-40-8 -
 153439-40-8 98%View Details
153439-40-8 98%View Details
153439-40-8 -
 Fexofenadine Hydrochloride CAS 153439-40-8View Details
Fexofenadine Hydrochloride CAS 153439-40-8View Details
153439-40-8 -
 Fexofenadine CAS 153439-40-8View Details
Fexofenadine CAS 153439-40-8View Details
153439-40-8 -
 Fexofenadine hydrochloride CAS 153439-40-8View Details
Fexofenadine hydrochloride CAS 153439-40-8View Details
153439-40-8
