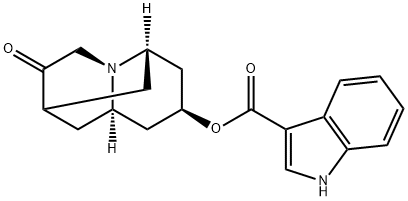
Dolasetron mesylate
Synonym(s):Anzemet hydrate;Dolasetron mesylate monohydrate;Dolasetron methanesulfonate;Dolasetron methanesulfonate hydrate
- CAS NO.:115956-13-3
- Empirical Formula: C20H26N2O7S
- Molecular Weight: 438.49
- MDL number: MFCD01718979
- EINECS: 601-404-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
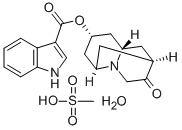
What is Dolasetron mesylate?
Description
Dolasetron was launched as Anzemet in Australia and the US for the prevention of nausea and vomiting in chemotherapy patients. It is a highly potent and very selective antagonist of 5-HT3 receptors ; it is the sixth in this class of compounds to be marketed for the treatment of chemotherapy-induced emesis. The last two approved in this class were Nazasetron (1994) and Ramosetron (1996). Anzemet was prepared by a seven step sequence from a cyclopentenecarboxylic ester via a Robinson-Schopf cyclisation of a dialdehyde into a key 9-azabicyclo[3.3.l]nonan-3-one. In a clinical study with 164 cancer patients treated with Dolasetron mesylate prior to Cisplatin, single doses of 10- 50 mg achieved major control of nausea and emesis in 73% of subjects and were well tolerated. Results from pharmacokinetic studies in humans showed that the clinical effects and duration of action seem to be due mainly to a major plasma metabolite rapidly formed and very potent itself, the (+) enantiomeric alcohol obtained by enzymatic reduction of the cyclic ketone.
Originator
Hoechst Marion Roussel (Germany)
The Uses of Dolasetron mesylate
Dolasetron Mesylate acts as a bridged pseudopelletierine derivative; specific serotonin (5HT3) receptor antagonist. Antiemetic.
The Uses of Dolasetron mesylate
Nausea in chemotherapy;5-HT3 antagonist
The Uses of Dolasetron mesylate
Antidepressant
brand name
Anzemet (Sanofi Aventis.
Veterinary Drugs and Treatments
Dolasetron may be effective in treating severe nausea and vomiting in dogs and cats, particularly if caused by cancer chemotherapy drugs. Because it is given once a day, the injectable form of dolasetron is often preferred over ondansetron, a similarly effective antiemetic. However, for oral use in small animals, dolasetron tablets are too large (50 and 100 mg) to be practically administered.
Properties of Dolasetron mesylate
| Melting point: | 278° |
| storage temp. | Sealed in dry,2-8°C |
| solubility | H2O: >30mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 115956-13-3(CAS DataBase Reference) |
Safety information for Dolasetron mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dolasetron mesylate
Dolasetron mesylate manufacturer
AKASH PHARMA EXPORTS
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Dolasetron mesylate 98%View Details
Dolasetron mesylate 98%View Details -
 115956-13-3 98%View Details
115956-13-3 98%View Details
115956-13-3 -
 Dolasetron Mesylate 99%View Details
Dolasetron Mesylate 99%View Details -
 Dolasetron mesylate hydrate CAS 115956-13-3View Details
Dolasetron mesylate hydrate CAS 115956-13-3View Details
115956-13-3 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0