Pazufloxacin mesilate
- CAS NO.:163680-77-1
- Empirical Formula: C17H19FN2O7S
- Molecular Weight: 414.4053632
- MDL number: MFCD00913262
- EINECS: 634-946-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
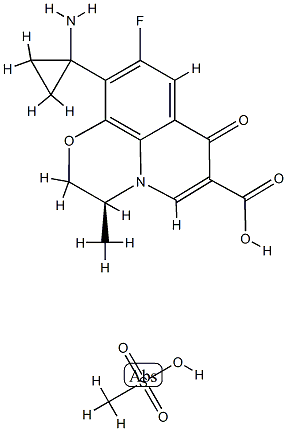
What is Pazufloxacin mesilate?
Description
This fluoroquinolone was co-developed by Toyama and Mitsubishi Pharm and was launched for the intravenous therapy of respiratory, urinary, surgical, gynecological and systemic infections.
Description
Pazufloxacin is a broad-spectrum synthetic fluoroquinolone antibiotic. It is active against Gram-negative and Gram-positive bacteria, including clinical isolates of E. coli, K. pneumoniae, methicillin-susceptible and -resistant S. aureus, and methicillin-susceptible and -resistant S. epidermidis (MIC90s = 0.05, 0.1, 0.39, 12.5, 0.39, and 6.25 μg/ml, respectively). It inhibits E. coli, P. aeruginosa, and S. aureus DNA gyrase (IC50s = 0.88, 1.9, and 10.2 μg/ml, respectively) and S. aureus topoisomerase IV (IC50 = 24.2 μg/ml) in cell-free assays. In vivo, pazufloxacin is active against systemic E. coli, K. pneumoniae, and methicillin-resistant S. aureus infections in mice (ED50s = 0.15, 5.5, and 4.5 mg/kg, respectively).
Chemical properties
Crystalline Solid
The Uses of Pazufloxacin mesilate
A fluorinated quinolone antibiotic. Antibacterial.
What are the applications of Application
Pazufloxacin Mesylate is a fluoroquinolone antimicrobial agent
Synthesis
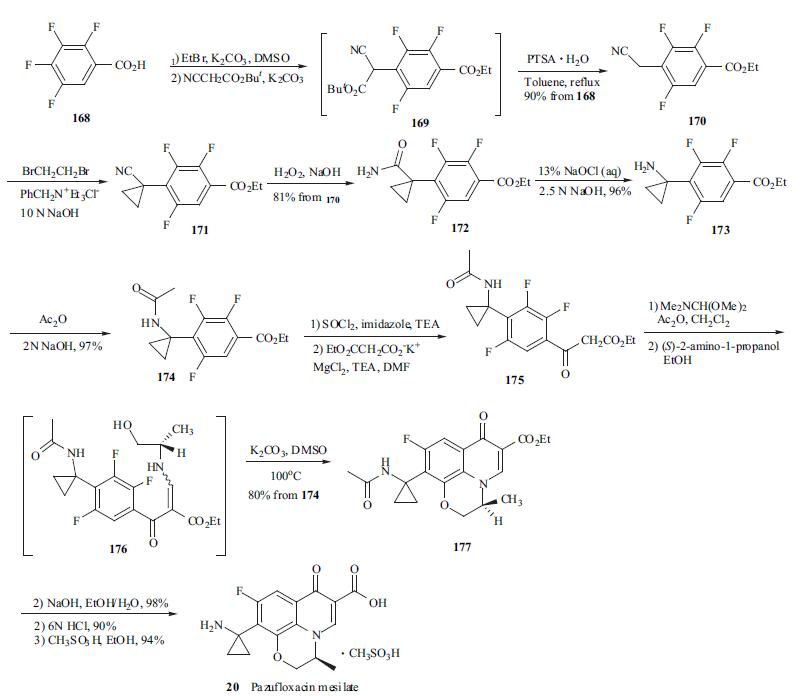
Pazufloxacin Mesilate is elegantly synthesized from commercially available 2,3,4,5-tetrafluorobenzoic acid (168) by an 11-step process with an overall yield 48% [68]. Starting material 168 was first treated with ethyl bromide and then with t-butyl cyanoacetate in the presence of potassium carbonate in DMSO in one flask to give acylated cyanoacetate 169. Intermediate 169 thus obtained without purification was refluxed in toluene with p-TSA to yield 4- cyanomethylbenzoate 170 in 90% yield from 168. Cyclopropanation at the benzylic position of 170 was performed by |á,|á-dialkylation with two equiv. of 1,2- dibromoethane under phase-transfer conditions to give cyanocyclopropyl compound 171. Cyano compound 171 was subjected to hydration with alkaline H2O2 to afford carboxamide 172 in 81% yield from 170. Subsequently, carboxamide 172 was treated with NaOCl for Hofmann rearrangement to give primary amine 173, which was protected as its N-acetyl derivative 174 for the next reaction. Treatment of 174 with imidazole in the presence of thionyl chloride and TEA generated an imidazolide intermediate, which was converted to |?-keto ester 175 by reacting with potassium ethyl malonate and MgCl2. Enamine 176 was obtained without purification by successive treatment of 175 with DMF-dimethylacetal and (S)-(+)-2-aminopropanol. Crude 176 was heated in DMSO in the presence of potassium carbonate to efficiently give tricycle product 177 in 80% yield from 174. Finally, the ethyl ester and acetamide in 177 were hydrolyzed under basic and acidic conditions, respectively, to give the free amine. Pazufloxacin mesilate (20) was obtained in 94% yield by treatment of its corresponding free amine with methanesulfonic acid in ethanol.
Properties of Pazufloxacin mesilate
| Melting point: | >255°C (dec.) |
| alpha | D20 -64.2° (c = 1 in 1.0N NaOH) |
| storage temp. | -20°C Freezer |
| solubility | Aqueous Base (Slightly), DMSO (Slightly) |
| form | Solid |
| color | Off-White to Pale Yellow |
| CAS DataBase Reference | 163680-77-1(CAS DataBase Reference) |
Safety information for Pazufloxacin mesilate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pazufloxacin mesilate
Pazufloxacin mesilate manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 163680-77-1 Pazufloxacin mesilate 98%View Details
163680-77-1 Pazufloxacin mesilate 98%View Details
163680-77-1 -
 Pazufloxacin mesylate 98% CAS 163680-77-1View Details
Pazufloxacin mesylate 98% CAS 163680-77-1View Details
163680-77-1 -
 Pazufloxacin Mesylate CAS 163680-77-1View Details
Pazufloxacin Mesylate CAS 163680-77-1View Details
163680-77-1 -
 Pazufloxacin mesylate 98% (HPLC) CAS 163680-77-1View Details
Pazufloxacin mesylate 98% (HPLC) CAS 163680-77-1View Details
163680-77-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1