Tropisetron
- CAS NO.:89565-68-4
- Empirical Formula: C17H20N2O2
- Molecular Weight: 284.35
- MDL number: MFCD00864399
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
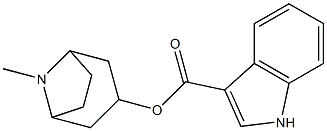
What is Tropisetron?
Absorption
The absorption of tropisetron from the gastrointestinal tract is rapid (mean half-life of about 20 minutes) and nearly complete (more than 95%). Due to first-pass metabolism in the liver, the absolute bioavailability of a 5 mg oral dose is 60%. The peak plasma concentration is attained within three hours.
Toxicity
LD50: 265 mg/kg (Rat, oral).
Description
Tropisetron is the third selective 5HT3-antagonist, after ondansetron and granisetron, to be introduced for the management of nausea and vomiting induced by cancer chemotherapy. Similar to the other two 5HT3-antagonists, vopisetron is superior to metoclopramide. The compound has also been reported to have antiarrhythmic activity.
Originator
Sandoz (Switzerland)
The Uses of Tropisetron
A substance that is used to prevent nausea and vomiting caused by chemotherapy.
Indications
For the prevention of nausea and vomiting induced by cytotoxic therapy and postoperative.
Background
Tropisetron is an indole derivative with antiemetic activity. As a selective serotonin receptor antagonist, tropisetron competitively blocks the action of serotonin at 5HT3 receptors, resulting in suppression of chemotherapy- and radiotherapy-induced nausea and vomiting.
Tropisetron appears to be well tolerated with the most frequently reported adverse effect being headache. Extrapyramidal side effects are rare upon using tropisetron.
Definition
ChEBI: An indolyl carboxylate ester obtained by formal condensation of the carboxy group of indole-3-carboxylic acid with the hydroxy group of tropine.
brand name
Novaban
Hazard
Human systemic effects.
Metabolism
The metabolism of tropisetron occurs by hydroxylation at the 5, 6 or 7 positions of its indole ring, followed by a conjugation reaction to the glucuronide or sulphate with excretion in the urine or bile (urine to faeces ratio 5:1). The metabolites have a greatly reduced potency for the 5-HT3 receptor and do not contribute to the pharmacological action of the drug.
Properties of Tropisetron
| Melting point: | 201-202 °C |
| Boiling point: | 448.5±35.0 °C(Predicted) |
| Density | 1.26 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: soluble |
| form | solid |
| pka | 15.38±0.30(Predicted) |
| color | white |
| CAS DataBase Reference | 89565-68-4(CAS DataBase Reference) |
Safety information for Tropisetron
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tropisetron
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
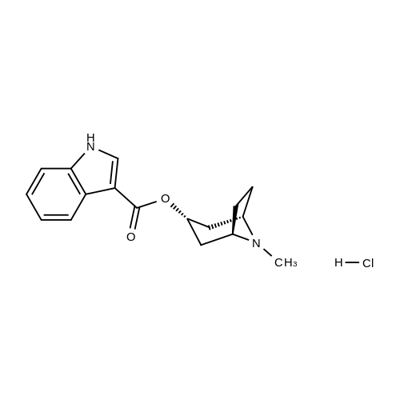
![8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, hydrochloride (1:1), (3-endo)-](https://img.chemicalbook.in/)



You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
