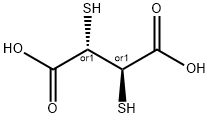
DL-Dithiothreitol
Synonym(s):DTT;1,4-Dithiothreitol;Cleland’s reagent;Dithiothreitol, DTT;(±)-threo-1,4-Dimercapto-2,3-butanediol solution
- CAS NO.:3483-12-3
- Empirical Formula: C4H10O2S2
- Molecular Weight: 154.25
- MDL number: MFCD00004877
- EINECS: 222-468-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
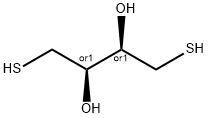
What is DL-Dithiothreitol?
Chemical properties
Dithiothreitol (DTT), also known as Cleland's reagent, is a small-molecule redox reagent . Its oxidized form is a disulfide-bonded 6-membered ring. DTT has an epimeric ('sister') compound, dithioerythritol (DTE).
DTT is a white crystalline powder. It is highly soluble in water (clear solution, OD<0.05 at 0.02M), but also in ethanol, chloroform, ether and ethyl acetate.
DTT is an unusually strong reducing agent, with a redox potential of -0.33 V at pH 7. The pKa of thiol groups is typically ~8.3.
The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions.
Description
DL-Dithiothreitol is redox reagent also known as Cleland's reagent, after W. Wallace Cleland. The reagent is commonly used in its racemic form.
Chemical properties
DL-Dithiothreitol(DTT) is oxidized by air. So it is normally stored and handled under inert atmosphere to minimize oxidation. The rate of air-oxidation is slower at low temperatures. Oxidized DTT exhibits a strong absorbance peak at 280 nm. Since thiols are less nucleophilic than their conjugate bases, thiolates, DTT becomes a less potent nucleophile as the pH falls. DTT's half-life is 40 hours at pH 6.5 and 1.4 hours at pH 8.5 and 20 °C; its half-life decreases further as temperature increases. The presence of EDTA (ethylenediaminetetraacetic acid) to chelate divalent metal ions considerably extends the half-life of DTT in solution.
The Uses of DL-Dithiothreitol
DL-dithiothreitol (DTT) is a sulfhydryl compound that acts both as a reagent reducing disulfide bonds and as a protein denaturant on staphylococcal biofilm (Wu et al. 2011). Thanks to these properties, DTT is routinely used in clinical microbiology to liquefy respiratory specimens, but it has also been proven effective to detach biofilm from orthopaedic prosthesis (Drago et al. 2012, 2013). Since it is crucial to discriminate implant-related infections from aseptic loosening in orthopaedics, and because of the difficulty to diagnose subclinical infections, a prompt diagnosis of indwelling device-related infection is important for successful treatments (Borens et al. 2013). A fast microbiological diagnosis and bacterial identification should be recommended in order to set up a specific antimicrobial therapy. According to this strategy, the bacterial detachment from biofilm on explants should be accelerated by supporting the pathogen viability.
The Uses of DL-Dithiothreitol
Dithiothreitol is a redox reagent commonly used as a reducing agent for thiolated DNA. Dithiothreitol is also used to reduce the disulfide bonds of proteins.
Definition
ChEBI: 1,4-dithiothreitol is the threo-diastereomer of 1,4-dimercaptobutane-2,3-diol. It has a role as a reducing agent, a chelator and a human metabolite. It is a dithiol and a 1,4-dimercaptobutane-2,3-diol.
What are the applications of Application
Dithiothreitol (DTT) is a water-soluble reducing reagent used for various applications in biotechnology, biology and biochemistry :
reduces quantitatively disulfides, generating sulfhydryls (used typically at 1-10mM for protein SS reduction)
reduction of proteins before SDS-PAGE analysis, studies of protein structure and function (Kaji 1993)
keep sulfhydryl groups of biomolecules in the reduced state - protects biomolecules in various applications (enzymes or receptors, living cells under ionizing radiations)
prevents the fading of fluorescence such as FITC labeled conjugates (Picciolo 1984)
General Description
1,4-Dithiothreitol (DTT) is the threo isomer of 2,3-dihydroxy-1,4-dithiolbutane, and an isomer of 1,4-dithioerythritol. DTT is used in molecular biology to maintain sulfhydryl (-SH) groups in the reduced state and for quantitative reduction of disulfide (-S-S-) groups, as described by Cleland in his pioneering publication from the 1960's. Its usefulness as an reducing agent stems from its water solubility and reduced odor compared to previous thiol compounds.
DTT is oxidized to the cyclic disulfide, and thereby ensures the reduction of other disulfides in solution. The disulfide reduction is complete in minutes at pH 8. DTT is less pungent and less toxic than 2-mercaptoethanol. Typically, a 7-fold lower concentration of DTT (100 mM) is required compared to 2-mercaptoethanol (5% v/v, 700 mM).
Biochem/physiol Actions
DL-Dithiothreitol denatures proteins by reducing the disulphide bonds and reducing them to SH groups. It also prevents intramolecular and intermolecular disulfide bond formation between cysteine residues of proteins.
Toxicology
The thiol-containing compound Dithiothreitol (DTT) is toxic to cultured cells by inducing the generation of reactive oxygen species that ultimately cause cell death. DTT treatment triggers the transcription of the canonical apoptosis regulators grim, hid, and rpr at low amounts. The amplitude of this induction declines with elevating DTT amounts. Through Live microscopy, Wang et al. have observed apoptotic cells, especially in the gut of DTT-treated flies. In parallel, low DTT amounts also activate the expression of the cuticle barrier component gene snsl. This indicates that a physical defense response is launched upon DTT contact. This combined measure is seemingly successful in preventing fly death. The expression of several known detoxification genes, including cyp6a2, cyp6a8, cyp12d1, and GstD2, is also enhanced through DTT contact[1].
References
[1] Yiwen Wang . “Toxicity of Dithiothreitol (DTT) to Drosophila melanogaster.” Toxicology Reports 8 (2021): Pages 124-130.
Synthesis
The most widely used DL-Dithiothreitol (DTT) synthetic method is to be raw material with Soviet Union's moss sugar alcohol, use potassium permanganate oxidation earlier, obtains the sulfo-intermediate again under the thioacetic acid effect, and this sulfo-intermediate of hydrolysis obtains DTT at last.
Yu discloses a synthesizing method of DL-Dithiothreitol.1,4-butylene glycol is used as an initiating raw material and undergoes an addition reaction with bromine at first to obtain 2,3-dibromo-1,4-butylene glycol; hydrolyzation under the catalyzation of alkali is carried out to obtain dioxirane; addition reaction with thioacetic acid is carried out to obtain dithiothreitol diacetate; and finally hydrolyzation under the catalyzation of alkali is carried out to obtain the DL-Dithiothreitol.
Storage
Store lyophilized at 4°C, desiccated. In lyophilized form, the chemical is stable for 12 months. Once in solution, store at -20°C and use within 3 months to prevent loss of potency. Aliquot to avoid multiple freeze/thaw cycles.
DTT solutions should be prepared fresh daily. If improperly stored (including room temperature and solution forms) its reducing ability may be reduced. Exposure to air should be minimized, even though DTT has a lower tendency to be oxidized directly by air than other reducing agents.
Recorded half-life of DTT solutions at various pH and temperatures (all are in M potassium phosphate buffer):
pH 6.5 @ 20°C= 40 hours
pH 7.5 @ 20°C = 10 hours
pH 8.5 @ 20°C = 1.4 hours
pH 8.5 @ 0°C = 11 hours
pH 8.5 @ 40°C = 0.2 hours
pH 8.5 @20°C (+0.1 mM Cu2+) = 0.6 hours
pH 8.5 @20°C (+0.1 mM EDTA) = 4 hours
Properties of DL-Dithiothreitol
| Melting point: | 41-44 °C(lit.) |
| Boiling point: | 125 °C |
| alpha | -0.2~+0.2°(20℃/D)(c=5,H2O) |
| Density | 1.04 g/mL at 20 °C |
| vapor density | 5.3 (vs air) |
| vapor pressure | 0.019-0.29Pa at 20-50℃ |
| refractive index | 1.5200 (estimate) |
| Flash point: | >230 °F |
| storage temp. | -20°C (or 4°C short term). |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Powder |
| pka | pK1:8.9 (25°C) |
| color | White |
| PH | 4.0-6.0 (20-25℃, 0.1m in H2O) |
| Odor | Unpleasant Odor |
| PH Range | 4 - 6 at 15,4 g/l at 25 °C |
| Water Solubility | freely soluble |
| Sensitive | Air Sensitive |
| λmax | λ: 260 nm Amax: 0.400 λ: 280 nm Amax: 0.100 |
| Merck | 14,3376 |
| BRN | 1719757 |
| Stability: | Stability Stable, but heat sensitive. Incompatible with strong oxidizing agents. Keep frozen at -20 to -10 C. |
| CAS DataBase Reference | 3483-12-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,3-Butanediol, 1,4-dimercapto-, (r*,r*)-(3483-12-3) |
| EPA Substance Registry System | 1,4-Dithiothreitol (3483-12-3) |
Safety information for DL-Dithiothreitol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DL-Dithiothreitol
| InChIKey | VHJLVAABSRFDPM-UHFFFAOYSA-N |
DL-Dithiothreitol manufacturer
KP INNOVATIVE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 DL-1, 4-Dithiothreitol CAS 3483-12-3View Details
DL-1, 4-Dithiothreitol CAS 3483-12-3View Details
3483-12-3 -
 DITHIOTHREITOL (DTT) 99%View Details
DITHIOTHREITOL (DTT) 99%View Details -
 DL-Dithiothreitol (DTT) extrapure CAS 3483-12-3View Details
DL-Dithiothreitol (DTT) extrapure CAS 3483-12-3View Details
3483-12-3 -
 DL-Dithiothreitol (DTT) ExiPlus CAS 3483-12-3View Details
DL-Dithiothreitol (DTT) ExiPlus CAS 3483-12-3View Details
3483-12-3 -
 DITHIOTHREITOL Extra Pure CASView Details
DITHIOTHREITOL Extra Pure CASView Details -
 DL-Dithiothreitol (DTT) for molecular biology CAS 3483-12-3View Details
DL-Dithiothreitol (DTT) for molecular biology CAS 3483-12-3View Details
3483-12-3 -
 DL-Dithiothreitol (DTT) ,98% CAS 3483-12-3View Details
DL-Dithiothreitol (DTT) ,98% CAS 3483-12-3View Details
3483-12-3 -
 DL-Dithiothreitol 98+View Details
DL-Dithiothreitol 98+View Details
'3483-12-3
