Succimer
Synonym(s):2,3-Dithio-meso-tartaric acid;DIM-SA;DMS;DMSA;Succimer
- CAS NO.:304-55-2
- Empirical Formula: C4H6O4S2
- Molecular Weight: 182.22
- MDL number: MFCD00064799
- EINECS: 206-155-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
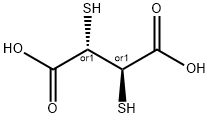
What is Succimer?
Absorption
Rapid but variable.
Toxicity
Oral LD50 in mice is over 5011 mg/kg. Doses of 2300 mg/kg in the rat and 2400 mg/kg in the mouse produced ataxia, convulsions, labored respiration and frequently death. No case of overdosage has been reported in humans. Limited data indicate that succimer is dialyzable.
Description
Succimer is a newly available oral chelator of lead and other heavy metals. The compound is indicated for the treatment of lead poisoning in children. Succimer does not bind iron, calcium or magnesium and preferentially binds lead, mercury and arsenic over copper or zinc. The metal-bound succimer is excreted in the urine. The drug may also be effective in adults.
Chemical properties
white or off-white powder
Originator
Johnson and Johnson (J&J) (U.S.A.)
The Uses of Succimer
chelating agent, antihypertensive
The Uses of Succimer
meso-2,3-Dimercaptosuccinic Acid is used as a chelating agent and masking agent for cadmium in EDTA titration of zinc.
The Uses of Succimer
chelating agent, treatment of lead poisoning
The Uses of Succimer
A water-soluble chelating agent.
Indications
For the treatment of lead poisoning in pediatric patients with blood lead levels above 45 μg/dL. May also be used to treat mercury or arsenic poisoning.
Background
A mercaptodicarboxylic acid used as an antidote to heavy metal poisoning because it forms strong chelates with them.
What are the applications of Application
DMSA (Meso-2,3-dimercaptosuccinic acid) is a water-soluble chelating agent
Definition
ChEBI: A sulfur-containing carboxylic acid that is succinic acid bearing two mercapto substituents at positions 2 and 3. A lead chelator used as an antedote to lead poisoning.
Preparation
Isolate
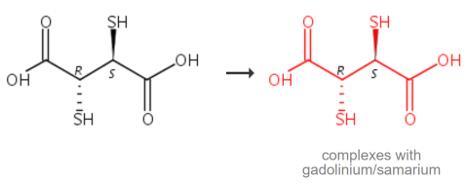
the solid complexes of trivalent lanthanide complexes with
meso-2,3-Dimercaptosuccinic Acid, furan-2-carboxylic acid,
meso-2,3-dimercaptosuccinic acid and sarcosine from the mixture of
equimolar solutions of metal nitrates and ligands. Adjust the pH of the
mixture to 7 by adding dilute solution of KOH. Reflux the mixture in
ethanol (15-20 ml) for 3-4 hours on a steam bath. The clear solution
gives a solid mass on cooling, filter through G4 glass crucible and wash
several times with the mixture of doubly distilled water and alcohol.
Recrystallise it to obtain pure crystal and dry at 60 ?? -70 ??C [1].
brand name
Chemet (Ovation).
Pharmaceutical Applications
Dimercaptosuccinic acid (DMSA) is a modification of BAL containing two thiol groups, which are responsible for the unpleasant
smell, and two carboxylic acid groups. DMSA is also known under the name Succimer. It chemical name
is meso-2,3-dimercaptosuccinic acid and the chemical formula is HO2CCH(SH)CH(SH)CO2H. There are
two diastereomeric forms, meso and the chiral DL forms, with the meso isomer being used as chelating agent.
DMSA was developed in the 1960s and replaced BAL and edetate in some countries for the treatment
of lead, arsenic and mercury poisoning. Furthermore, the dimethylester modification of DMSA has been
successfully used for the treatment of heavy-metal poisoning.
Pharmaceutical Applications
The unlicensed drug Succimer (DMSA, meso-2,3-dimercaptosuccinic acid) may be valuable in the treatment of most forms of heavy-metal poisoning including lead, arsenic and mercury. These and other chelating agents such as unithiol (DMPS, 2,3-dimercapto-1-propanesulfonic acid) and α-lipoic acid (ALA) are also used in alternative medicine, which has led to much criticism and discussion. So far, no medical study has proven the effectiveness of chelation therapy for any clinical application other than heavy-metal poisoning.
Pharmacokinetics
Succimer is an orally active, heavy metal chelating agent. It forms water soluble chelates and, consequently, increases the urinary excretion of lead. Succimer is not to be used for prophylaxis of lead poisoning in a lead-containing environment. In addition, the use of succimer should always be accompanied by identification and removal of the source of the lead exposure.
Veterinary Drugs and Treatments
In veterinary medicine, succimer may be useful for the oral treatment of lead poisoning in small animals (including birds). Potentially, it also may be of benefit for the treatment of other toxic heavy metals such as arsenic or mercury, but more research must be done before this can be recommended.
Metabolism
Chemical analysis of succimer and its metabolites (primarily mixed disulfides of L-cysteine) in the urine showed that succimer was rapidly and extensively metabolized however the specific site of biotransformation is not known.
Purification Methods
Purify the acid by dissolving it in NaOH and precipitating with dilute HCl, drying and recrystallising from MeOH. IR has at 2544 (SH) and max 1689 (CO2H) cm-1 . The bis-S-acetyl derivative has m 183-185o (from EtOAc or Me2CO), and its Me ester has m 119-120o (from pet ether) [Gerecke et al. Helv Chim Acta 44 957 1961, Owen & Sultanbawa J Chem Soc 3112 1949]. [Beilstein 3 III 1033.]
Properties of Succimer
| Melting point: | 196-198 °C (dec.)(lit.) |
| Boiling point: | 285.62°C (rough estimate) |
| Density | 1.439 (estimate) |
| refractive index | 1.5220 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Sparingly), Methanol (Slightly, Heated) |
| form | Powder |
| pka | pK1:2.71;pK2:3.48;pK3:8.89(SH);pK4:10.79(SH) (25°C) |
| color | White to off-white |
| Water Solubility | Soluble in water, and ethanol (25 mg/ml, results in colorless to light yellow, clear). |
| Merck | 14,8865 |
| BRN | 1725150 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 304-55-2(CAS DataBase Reference) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dimercapto-, (2R,3S)-rel- (304-55-2) |
Safety information for Succimer
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Succimer
| InChIKey | ACTRVOBWPAIOHC-XIXRPRMCSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 meso-2,3-Dimercaptosuccinic Acid CAS 304-55-2View Details
meso-2,3-Dimercaptosuccinic Acid CAS 304-55-2View Details
304-55-2 -
 2,3-Dimercapto succinic acid+ CAS 304-55-2View Details
2,3-Dimercapto succinic acid+ CAS 304-55-2View Details
304-55-2 -
 Meso-2,3-dimercaptosuccinic acid 95% CAS 304-55-2View Details
Meso-2,3-dimercaptosuccinic acid 95% CAS 304-55-2View Details
304-55-2 -
 2,3-Dimercapto succinic acid CAS 304-55-2View Details
2,3-Dimercapto succinic acid CAS 304-55-2View Details
304-55-2 -
 meso-2,3-Dimercaptosuccinic acid CAS 304-55-2View Details
meso-2,3-Dimercaptosuccinic acid CAS 304-55-2View Details
304-55-2 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
