1,4-Butanediol
Synonym(s):1,4-Butanediol;1,4-Butylene glycol;Tetramethylene glycol
- CAS NO.:110-63-4
- Empirical Formula: C4H10O2
- Molecular Weight: 90.12
- MDL number: MFCD00002968
- EINECS: 203-786-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
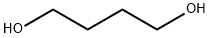
What is 1,4-Butanediol?
Chemical properties
1,4-butanediol (1,4-BD) is a colorless, viscous liquid derived from butane by placement of alcohol groups at each end of its molecular chain and is one of four stable isomers of butanediol.the hydroxyl function of each end group of the Butanediol reacts with different mono- and bifunctional reagents: for example with dicarboxylic acids to polyesters, with diisocyanates to polyurethanes, or with phosgene to polycarbonates. 1.4-Butanediol (BDO) is a high-quality intermediate. BDO and its derivatives are widely used for producing plastics, solvents, electronic chemicals and elastic fibers. Additionally BDO is also a building block for the synthesis of polyesterpolyols and polyetherpolyols. BASF is the most significant producer of 1,4-Butanediol and its derivatives worldwide.
The Uses of 1,4-Butanediol
- Butanediol and its derivatives is used in a broad spectrum of applications in the chemical industry; amongst others in the manufacturing of technical plastics, polyurethanes, solvents, electronic chemicals and elastic fibres.
- 1,4-Butanediol is used in the synthesis of epothilones, a new class of cancer drugs. Also used in the stereoselective synthesis of (-)-Brevisamide.
- 1,4-Butanediol's largest use is within tetrahydrofuran (THF) production, used to make polytetramethylene ether glycol, which goes mainly into spandex fibers, urethane elastomers, and copolyester ethers.
- It is commonly used as a solvent in the chemical industry to manufacture gamma-butyrolactone and elastic fibers like spandex.
- It is used as a cross-linking agent for thermoplastic urethanes, polyester plasticizers, paints and coatings.
- It undergoes dehydration in the presence of phosphoric acid yielded teterahydrofuran, which is an important solvent used for various applications.
- It acts an intermediate and is used to manufacture polytetramethylene ether glycol (PTMEG), polybutylene terephthalate (PBT) and polyurethane (PU).
- It finds application as an industrial cleaner and a glue remover.
- 1,4-butanediol is also used as a plasticiser (e.g. in polyesters and cellulosics), as a carrier solvent in printing ink, a cleaning agent, an adhesive (in leather, plastics, polyester laminates and polyurethane footwear), in agricultural and veterinary chemicals and in coatings (in paints, varnishes and films).
The Uses of 1,4-Butanediol
butylene glycol is a solvent with good antimicrobial action. It enhances the preservative activity of parabens. Butylene glycol also serves as a humectant and viscosity controller, and to mask odor.
The Uses of 1,4-Butanediol
1,4-Butanediol is used to produce polybutyleneterephthalate, a thermoplastic polyester;and in making tetrahydrofuran, butyrolactones,and polymeric plasticizers.
Production Methods
Methods of manufacturing:
The most prevalent 1,4-BD production route worldwide is BASF's Reppe process, which reacts acetylene and formaldehyde. Acetylene reacts with two equivalents of formaldehyde to form 1,4-butynediol, also known as but-2-yne- 1,4-diol. Hydrogenation of 1,4-butynediol gives 1,4-butanediol. 1,4-BD is also made on a large industrial scale by continuous hydrogenation of the 2-butyne- 1,4-diol over modified nickel catalysts. The one-stage flow process is carried out at 80 - 160 deg C and 300 bar.
Mitsubishi uses a three-step process:
(1) the catalytic reaction of butadiene and acetic acid yields 1,4-diacetoxy-2-butene;
(2) subsequent hydrogenation gives 1,4-diacetoxybutane; and
(3) hydrolysis leads to 1,4-butanediol.
Definition
ChEBI: Butane-1,4-diol is a butanediol that is butane in which one hydrogen of each of the methyl groups is substituted by a hydroxy group. A colourless, water-miscible, viscous liquid at room temperature (m.p. 16℃) with a high boiling point (230℃), it is mainly used for the production of other organic chemicals, particularly the solvent oxolane (also known as tetrahydrofuran or THF). It has a role as a neurotoxin, a protic solvent and a prodrug. It is a butanediol and a glycol.
Synthesis Reference(s)
The Journal of Organic Chemistry, 36, p. 2018, 1971 DOI: 10.1021/jo00813a043
Tetrahedron Letters, 33, p. 6371, 1992 DOI: 10.1016/S0040-4039(00)60976-0
General Description
Odorless colorless liquid or solid (depending upon temperature).
Air & Water Reactions
Highly flammable. 1,4-Butanediol is hygroscopic. Water soluble.
Reactivity Profile
1,4-Butanediol is heat and light sensitive. 1,4-Butanediol reacts with acid chlorides, acid anhydrides and chloroformates; reacts with oxidizing agents and reducing agents. 1,4-Butanediol is incompatible with isocyanates and acids; also incompatible with peroxides, perchloric acid, sulfuric acid, hypochlorous acid, nitric acid, caustics, acetaldehyde, nitrogen peroxide and chlorine.
Hazard
Toxic by ingestion.
Health Hazard
Ingestion of large amounts needed to produce any symptoms.
Health Hazard
The acute toxic effects are mild. 1,4-Butanediolis less toxic than its unsaturate analogs,butenediol and the butynediol. The oralLD50 value in white rats and guinea pigsis ~2 mL/kg. The toxic symptoms fromingestion may include excitement, depressionof the central nervous system, nausea, anddrowsiness.
Fire Hazard
Nonflammable liquid, flash point (open cup) 121°C.
Safety Profile
A human poison by an unspecified route. Moderately toxic byingestion and intraperitoneal routes. Human systemic effects: altered sleep time. Combustible when exposed to heat or flame. To fight fire, use alcohol foam, mist, foam, CO2, dry chemical. Incompatible with oxidizing materials. When heated to decomposition it emits acrid smoke and fumes.
Solubility in water
1,4-Butanediol is soluble in water, alcohols, esters, ketones, glycol ethers and glycol ether acetates (Butanediols, Butenediol, and Butynediol).
Purification Methods
Distil the glycol and store it over Linde type 4A molecular sieves, or crystallise it twice from anhydrous diethyl ether/acetone, and redistil it. It has been recrystallised from the melt and doubly distilled in vacuo in the presence of Na2SO4. [Beilstein 1 IV 2515.]
Properties of 1,4-Butanediol
| Melting point: | 16 °C (lit.) |
| Boiling point: | 230 °C (lit.) |
| Density | 1.017 g/mL at 25 °C (lit.) |
| vapor density | 3.1 (vs air) |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | n |
| Flash point: | 135 °C |
| storage temp. | Store below +30°C. |
| form | Liquid |
| pka | 14.73±0.10(Predicted) |
| color | Clear colorless |
| Odor | Odorless |
| PH | 7-8 (500g/l, H2O, 20℃) |
| explosive limit | 1.95-18.3%(V) |
| Water Solubility | Miscible |
| Sensitive | Hygroscopic |
| BRN | 1633445 |
| Dielectric constant | 30.0(30℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, mineral acids, acid chlorides, acid anhydrides. |
| CAS DataBase Reference | 110-63-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Butanediol(110-63-4) |
| EPA Substance Registry System | 1,4-Butanediol (110-63-4) |
Safety information for 1,4-Butanediol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 1,4-Butanediol
| InChIKey | WERYXYBDKMZEQL-UHFFFAOYSA-N |
1,4-Butanediol manufacturer
Pat Impex
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




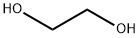
You may like
-
 1-4,BUTANEDIOL 99%View Details
1-4,BUTANEDIOL 99%View Details -
 1,4-Butanediol CAS 110-63-4View Details
1,4-Butanediol CAS 110-63-4View Details
110-63-4 -
 1,4-Butanediol, >99%, 50 litre drum for manufacturing of agrochemicalsView Details
1,4-Butanediol, >99%, 50 litre drum for manufacturing of agrochemicalsView Details
110-63-4 -
 1,4, Butane Diol (14bdo)View Details
1,4, Butane Diol (14bdo)View Details
110-63-4 -
 1,4 Butane Diol - Dairen Chemical Corp, Taiwan For Pharmaceuticals, 200 Kg DrumView Details
1,4 Butane Diol - Dairen Chemical Corp, Taiwan For Pharmaceuticals, 200 Kg DrumView Details
110-63-4 -
 Butylene GlycolView Details
Butylene GlycolView Details
107-88-0 -
 Butylene Glycol (1 3 Butylene Glycol) Cosmetic Grade High QUalityView Details
Butylene Glycol (1 3 Butylene Glycol) Cosmetic Grade High QUalityView Details
110-63-4 -
 1, 4 Butane DiolView Details
1, 4 Butane DiolView Details
110-63-4
