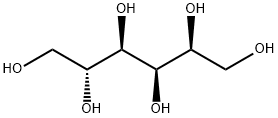
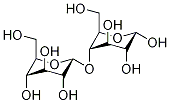
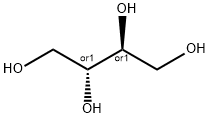
Maltitol
Synonym(s):4-O-α-Glucopyranosyl-D -sorbitol
- CAS NO.:585-88-6
- Empirical Formula: C12H24O11
- Molecular Weight: 344.31
- MDL number: MFCD00006600
- EINECS: 209-567-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24

What is Maltitol?
Description
Maltitol is a-D-glucopyranosyl-1.4-glucitol. The solubility in water is approximately 1,750 g/L at room temperature. Maltitol is stable under the common processing conditions of foods. In addition to dry maltitol several types of syrups are available.
Maltitol is, depending on the concentration, approximately 90 % as sweet as sucrose and noncariogenic.
In the European Union, maltitol is approved as E 965 for a large number of food applications. It is GRAS in the United States and also approved in many other countries.
Chemical properties
White or almost white, crystalline powder
Chemical properties
Maltitol is a-D-glucopyranosyl-1.4-glucitol. The solubility in water is approximately
1,750 g/L at room temperature. Maltitol is stable under the common processing
conditions of foods. In addition to dry maltitol several types of syrups are
available.
Maltitol is, depending on the concentration, approximately 90 % as sweet as
sucrose and noncariogenic .
In the European Union, maltitol is approved as E 965 for a large number of food
applications. It is GRAS in the United States and also approved in many other
countries.
Chemical properties
Maltitol occurs as a white, odorless, sweet, anhydrous crystalline powder. It is a disaccharide consisting of one glucose unit linked with one sorbitol unit via an α-(1→4) bond. The crystal structure is orthorhombic.
Chemical properties
Maltitol (ο-α-D-glucopyranosyl-(l-4)-sorbitol) is a disaccharide sugar alcohol derived from maltose by hydrogenation. On a commercial scale, maltitol is produced by hydrogenation of corn syrup with high maltose content that is prepared by enzymatic hydrolysis of starch. After purification and concentration of the hydrogenated syrup ("hydrogenated glucose syrup"), a crystalline product with a maltitol content of between 90 and 99% and small amounts of sorbitol and hydrogenated trisaccharides are obtained. Pure maltitol is about 0.8 times as sweet as sucrose. In vitro experiments with enzyme preparations, homogenates of the intestinal mucosa, and everted intestinal sacs have demonstrated that maltitol may be hydrolyzed to glucose and sorbitol (the former is absorbed and the latter is incompletely absorbed and is subject to microbial fermentation in the gut). The hydrolysis of maltitol proceeds at a slower rate than that of sucrose but faster than that of isomalt or lactitol. To determine the extent to which maltitol is hydrolyzed under in vivo conditions, gnotobiotic ratswere given doses of 1.5 g maltitol or maltose. Analysis of residual test substance in the gastrointestinal tract 60 to 120 minutes after dosing demonstrated that 69% of the maltitol and 99% of the maltose was hydrolyzed in the small intestine. In a study with germfree rats, 84% of an ingested maltitol dose disappeared from the gastrointestinal tract within 24 hr. Streptococcus mutans, Actinomyces viscosus, and some species of Lactobacillus ferment maltitol, but S. sanguis and S. mitior do not. Maltitol (10% solution) does not lower plaque pH below 5.7 in humans tested by plaque telemetry.
The Uses of Maltitol
Maltitol is a polyhydric alcohol (polyol) produced by hydrogenation of maltose. it is approximately 90% as sweet as sucrose, has good stability, and is nonhygroscopic. uses include chewing gum, dry nut bakery products, and chocolate.
The Uses of Maltitol
Sweetener.
The Uses of Maltitol
Maltol is a flavor enhancer used as a synthetic flavoring substance, the function of which is related to ethyl . It occurs naturally in chicory, cocoa, coffee, and cereals. It does not contribute a flavor of its own, but modifies the inherent flavors. As compared to ethyl , it is one-half to one-sixth as effective. It is less soluble, having a solubility of 1 g in 82 ml of water at 25°c. It has a melting range of 160–164°c. It is used to enhance the flavor and aroma of fruit, vanilla, and chocolate flavored foods and beverages. It is also used in beverages and desserts with a typical usage range of 10–200 ppm.
What are the applications of Application
Maltitol is a sugar substitute which possess a weak genotoxic effect
Definition
ChEBI: An alpha-D-glucoside consisting of D-glucitol having an alpha-D-glucosyl residue attached at the 4-position. Used as a sugar substitute.
Production Methods
Maltitol is produced by chemical hydrogenation of maltose, which can be obtained by enzymatic degradation of starch under conditions similar to those used for other starch hydrolysates such as glucose. The Starting material can be the different commercially available starches including corn, potato, and others. A partially degraded starch, which can be obtained by treatment with diluted hydrochloric or sulphuric acid and subsequent neutralization or with heat-stable a-amylase, is then subjected to enzyme treatment for further degradation to maltose-rich products.
Enzymes used for maltose production are b-amylases, fungal a-amylases, a-1.6- glucosidases, maltogenic amylases, and debranching enzymes, preferably with high temperature optimum.
Production Methods
Maltitol is obtained from hydrogenated maltose syrup. Starch is hydrolyzed to yield a high-concentration maltose syrup, which is hydrogenated with a catalyst. After purification and concentration, the syrup is crystallized.
Biotechnological Production
Maltitol is produced by chemical hydrogenation of maltose, which can be obtained
by enzymatic degradation of starch under conditions similar to those used for other
starch hydrolysates such as glucose. The Starting material can be the different
commercially available starches including corn, potato, and others. A partially
degraded starch, which can be obtained by treatment with diluted hydrochloric or
sulphuric acid and subsequent neutralization or with heat-stable a-amylase, is then
subjected to enzyme treatment for further degradation to maltose-rich products.
Enzymes used for maltose production are b-amylases, fungal a-amylases, a-1.6-
glucosidases, maltogenic amylases, and debranching enzymes, preferably with
high temperature optimum.
Examples can be found in patent applications for processes for production of
maltose and maltitol.
General Description
Maltitol is a low-caloric artificial sweetener, consisting of a sugar alcohol (polyol),
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Maltitol is widely used in the pharmaceutical industry in the formulation of oral dosage forms. It is a noncariogenic bulk sweetener, approximately as sweet as sucrose, well adapted as a diluent for different oral dosage forms, wet granulation, and sugarfree hard coating.
Biochem/physiol Actions
Sugar substitute with a weak genotoxic effect.
Safety
Maltitol is used in oral pharmaceutical formulations, confectionery,
and food products, and is considered to be noncariogenic. It is
generally regarded as a nontoxic, nonallergenic, and nonirritant
material.
Digestion of maltitol follows two different metabolic pathways:
absorption in the small intestine and fermentation in the large
intestine (colon). These two metabolic pathways must thus be
considered when evaluating the energy value.
The hydrolysis of maltitol in the small intestine releases sorbitol
and glucose. Glucose is actively transported and rapidly absorbed,
whereas sorbitol absorption is passive. The nonabsorbed sorbitol and nonhydrolyzed maltitol are fermented by the microflora in the
colon. The relative importance of the two absorption pathways
depends on numerous individual factors and is related to the
quantity of maltitol ingested. Excessive oral consumption (>50 g
daily) may cause flatulence and diarrhea.
Maltitol exhibits a low glycemic index and can therefore, under
medical supervision, have a place in the diet of diabetic patients. The
intake of maltitol must be taken into account for the calculation of
the daily glucidic allowance.
The WHO, in considering the safety of maltitol, did not set a
value for the acceptable daily intake since the levels used in food to
achieve a desired effect were not considered a hazard to health.
Storage
Maltitol has good thermal and chemical stability. When it is heated at temperatures above 200°C, decomposition begins (depending on time, temperature, and other prevailing conditions). Maltitol does not undergo browning reactions with amino acids, and absorbs atmospheric moisture only at relative humidities of 89% and above, at 20°C.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in oral pharmaceutical formulations. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Maltitol
| Melting point: | 149-152 °C (lit.) |
| Boiling point: | 399.42°C (rough estimate) |
| alpha | [α]D20 +106~+108゜ (c=0.8, H2O) |
| Density | 1.3863 (rough estimate) |
| vapor pressure | 0.001Pa at 20℃ |
| refractive index | 105 ° (C=10, H2O) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, practically insoluble in anhydrous ethanol. |
| pka | 12.84±0.70(Predicted) |
| form | Crystalline Powder |
| color | White |
| Water Solubility | Soluble in water. Slightly soluble in ethanol. |
| BRN | 89983 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 585-88-6(CAS DataBase Reference) |
| EPA Substance Registry System | D-Glucitol, 4-O-.alpha.-D-glucopyranosyl- (585-88-6) |
Safety information for Maltitol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Maltitol
Maltitol manufacturer
Clickchem Research LLP
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Maltitol CAS 585-88-6View Details
Maltitol CAS 585-88-6View Details
585-88-6 -
 Maltitol CAS 585-88-6View Details
Maltitol CAS 585-88-6View Details
585-88-6 -
 Maltitol 98% (GC) CAS 585-88-6View Details
Maltitol 98% (GC) CAS 585-88-6View Details
585-88-6 -
 Maltitol 95.00% CAS 585-88-6View Details
Maltitol 95.00% CAS 585-88-6View Details
585-88-6 -
 Maltitol CAS 585-88-6View Details
Maltitol CAS 585-88-6View Details
585-88-6 -
 Maltitol CAS 585-88-6View Details
Maltitol CAS 585-88-6View Details
585-88-6 -
 Maltitol CAS 585-88-6View Details
Maltitol CAS 585-88-6View Details
585-88-6 -
 Solid Maltitol, 8002-74-2View Details
Solid Maltitol, 8002-74-2View Details
585-88-6
