Desoxycorticosterone
Synonym(s):DOC;11-Deoxycorticosterone;Desoxycortone;11-Desoxycorticosterone;21-Hydroxy-4-pregnene-3,20-dione
- CAS NO.:64-85-7
- Empirical Formula: C21H30O3
- Molecular Weight: 330.46
- MDL number: MFCD00003661
- EINECS: 200-596-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52

What is Desoxycorticosterone?
Chemical properties
white to creamy-white crystalline powder
The Uses of Desoxycorticosterone
A mineralocorticoid that occurs in adrenal cortex. It acts as a precursor to Aldosterone (A514700).
The Uses of Desoxycorticosterone
Antiinflammatory;Corticoide
What are the applications of Application
21-Hydroxyprogesterone is a mineralocorticoid
Definition
ChEBI: A mineralocorticoid that is progesterone substituted at position 21 by a hydroxy group.
General Description
11-Deoxycorticosterone is a steroid hormone produced in the adrenal glands that acts as a precursor for the hormone aldosterone. Levels of 11-deoxycorticosterone are measured by LC-MS/MS to aid in diagnosing disorders of steroid synthesis, such as 11-hydroxylase deficiency and glucocorticoid responsive hyperaldosteronism. This Certified Spiking Solution? is suitable for use as starting material inlinearity standards, calibrators, and controls for numerous LC-MS/MS applications in endocrinology, clinical chemistry, and neonatal screening.
Hazard
Toxic.
Mechanism of action
Desoxycorticosterone causes an increase in reabsorption of sodium ions and excretion of potassium ions from the renal tubules, which leads to increased tissue hydrophilicity. This facilitates an elevated volume of plasma and increased arterial pressure. Muscle tonicity and work capability are increased. It is used for an insufficiency of function of the adrenal cortex, myasthenia, asthenia, adynamia, and overall muscle weakness.
Synthesis
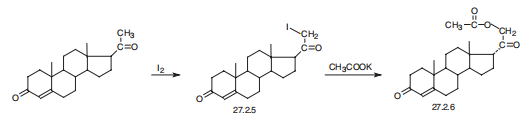
Desoxycorticosterone, 21-hydroxypregn-4-en-3,20-dione acetate (27.2.6), is synthesized in a number of ways, the easiest of which being iodination of progesterone at C21 in the methyl group, and subsequent reaction of the resulting iodo-derivative 27.2.5 with potassium acetate, which leads to formation of the desired desoxycorticosterone in the form of the acetate (27.2.6) .
Purification Methods
Crystallise 11-deoxycorticosterone from diethyl ether. [Schindler et al. Helv Chim Acta 24 360 1941, Steiger & Reichstein Helv Chim Acta 20 1164 1937.]
Properties of Desoxycorticosterone
| Melting point: | 138-144 °C |
| Boiling point: | 407.89°C (rough estimate) |
| alpha | 184 º (c=1, C2H5OH) |
| Density | 1.0998 (rough estimate) |
| refractive index | 1.5192 (estimate) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.98±0.10(Predicted) |
| color | White to Pale Yellow |
| Water Solubility | slightly soluble |
| Merck | 13,2917 |
| BRN | 2062123 |
| CAS DataBase Reference | 64-85-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Deoxycorticosterone(64-85-7) |
| EPA Substance Registry System | Deoxycorticosterone (64-85-7) |
Safety information for Desoxycorticosterone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Desoxycorticosterone
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(-)-2-[METHYLAMINO]-1-PHENYLPROPANE](https://img.chemicalbook.in/CAS/GIF/33817-09-3.gif)


You may like
-
 Desoxycorticosterone 97.00% CAS 64-85-7View Details
Desoxycorticosterone 97.00% CAS 64-85-7View Details
64-85-7 -
 11-Deoxycorticosterone solution CAS 64-85-7View Details
11-Deoxycorticosterone solution CAS 64-85-7View Details
64-85-7 -
 21-Hydroxyprogesterone CAS 64-85-7View Details
21-Hydroxyprogesterone CAS 64-85-7View Details
64-85-7 -
 21-HYDROXYPREGN-4-ENE-3,20-DIONE CAS 64-85-7View Details
21-HYDROXYPREGN-4-ENE-3,20-DIONE CAS 64-85-7View Details
64-85-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1