CORTICOSTERONE
Synonym(s):(11β)-11,21-Dihydroxypregn-4-ene-3,20-dione, Reichstein’s substance H;11β,21-Dihydroxy-4-pregnene-3,20-dione;11β,21-Dihydroxyprogesterone;4-Pregnene-11β,21-diol-3,20-dione;Corticosterone - CAS 50-22-6 - Calbiochem
- CAS NO.:50-22-6
- Empirical Formula: C21H30O4
- Molecular Weight: 346.47
- MDL number: MFCD00037715
- EINECS: 200-019-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is CORTICOSTERONE?
Description
Corticosterone is a steroid hormone produced in the cortex of the adrenal glands that binds to both glucocorticoid and mineralocorticoid receptors. It is produced in response to ACTH (corticotropic hormone) and is the precursor to aldosterone synthesis. Since the production of glucocorticoids is increased by stress, it is often used as a biomarker of stress. Plasma corticosterone levels have a circadian variation and corticosterone may be important in the regulation of the sleep-
Chemical properties
white to light yellow powder
The Uses of CORTICOSTERONE
Corticosterone: HBC complex has been used:
- in the intravenous and intraperitoneal administration of corticosterone to rats to test its effect on glucocorticoid and mineralocorticoid activity
- to induce acute stress in mice
- in Pulsatile treatment in mice to test its effect on clock gene period 1 transcription
The Uses of CORTICOSTERONE
Glucocorticoid; an intermediate in the biosynthesis of aldosterone, isolated from the adrenal cortex.
The Uses of CORTICOSTERONE
Corticosteroid is an activator of MCR.
What are the applications of Application
Corticosterone is an activator of MCRs and GRs
Definition
ChEBI: A 21-hydroxy steroid that consists of pregn-4-ene substituted by hydroxy groups at positions 11 and 21 and oxo groups at positions 3 and 20. Corticosterone is a 21-carbon steroid hormone of the corticosteroid type produced in the cortex of the adrenal glan s.
General Description
Corticosterone is a corticosteroid and aldosterone precursor produced in the adrenal glands. Serum corticosterone levels are measured by LC-MS/MS for newborn screening and diagnosis of 11-hydroxylase deficiency.
Biological Activity
Endogenous glucocorticoid that acts as an agonist at glucocorticoid and mineralocorticoid receptors.
Biochem/physiol Actions
The complex of corticosterone with 2-hydroxypropyl-β-cyclodextrin (HBC) improves water solubility. HBC is a carrier molecule. Corticosterone is a rodent-specific primary adrenal corticosteroid. It displays affinity towards the glucocorticoid and mineralocorticoid receptors.
storage
Room temperature
Purification Methods
Purify corticosterone by recrystallisation from Me2CO (trigonal plates), EtOH or isoPrOH. It has UV max at 240nm, and gives an orange-yellow solution with strong fluorescence on treatment with concentrated H2SO4. It is insoluble in H2O but soluble in organic solvents. [Reichstein & Euw Helv Chim Acta 2 1 1197 1938, 2 7 1287 1944; Mason et al. J Biol Chem 114 613 1936; ORD: Foltz et al. J Am Chem Soc 7 7 4359 1955; NMR: Shoolery & Rogers J Am Chem Soc 8 0 5121 1958.] The 21-O-acetyl derivative [1173-26-8] crystallises from Me2CO/Et2O with m 152.5-153o, [] D 20 +195o (c 0.6, Me2CO), and the 21-O-benzoyl derivative crystallises from AcOH/Et2O with m 201-202o [Reichstein Helv Chim Acta 2 0 953 1937]. [Beilstein 8 IV 2907.]
Properties of CORTICOSTERONE
| Melting point: | 179-183 °C(lit.) |
| alpha | D15 +223° (c = 1.1 in alc) |
| Boiling point: | 401.19°C (rough estimate) |
| Density | 1.0413 (rough estimate) |
| refractive index | 1.4430 (estimate) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly, Sonicated), Ethanol (Slightly, Sonicated), Methanol (Slig |
| form | White to tan crystalline powder |
| color | White to Pale Yellow |
| optical activity | [α]20/D +223±3°, c = 1% in ethanol |
| Water Solubility | 240.5mg/L(37 ºC) |
| Merck | 2538 |
| BRN | 2339601 |
| Stability: | Stable, but light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-22-6(CAS DataBase Reference) |
| EPA Substance Registry System | Corticosterone (50-22-6) |
Safety information for CORTICOSTERONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for CORTICOSTERONE
CORTICOSTERONE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
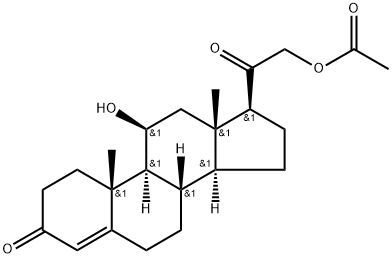
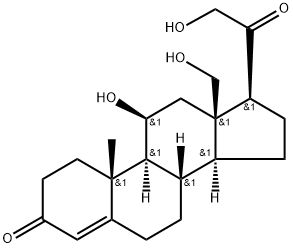
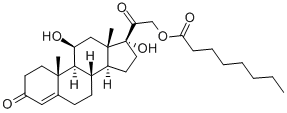
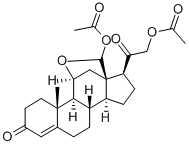
You may like
-
 50-22-6 Corticosterone 98%View Details
50-22-6 Corticosterone 98%View Details
50-22-6 -
 Corticosterone CAS 50-22-6View Details
Corticosterone CAS 50-22-6View Details
50-22-6 -
 Corticosterone CAS 50-22-6View Details
Corticosterone CAS 50-22-6View Details
50-22-6 -
 Corticosterone: HBC complex CAS 50-22-6View Details
Corticosterone: HBC complex CAS 50-22-6View Details
50-22-6 -
 Corticosterone CAS 50-22-6View Details
Corticosterone CAS 50-22-6View Details
50-22-6 -
 Corticosterone CAS 50-22-6View Details
Corticosterone CAS 50-22-6View Details
50-22-6 -
 Corticosterone CAS 50-22-6View Details
Corticosterone CAS 50-22-6View Details
50-22-6 -
 Corticosterone solution CAS 50-22-6View Details
Corticosterone solution CAS 50-22-6View Details
50-22-6