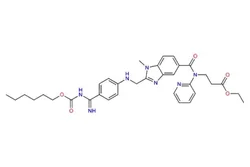
Dabigatran etexilate
- CAS NO.:211915-06-9
- Empirical Formula: C34H41N7O5
- Molecular Weight: 627.73
- MDL number: MFCD22422841
- EINECS: 606-722-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
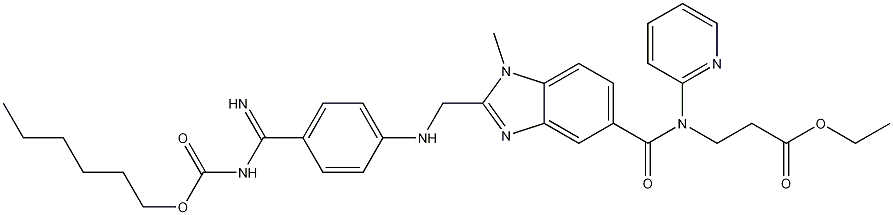
What is Dabigatran etexilate?
Absorption
Oral dabigatran has a bioavailability of 3-7%, although the relative bioavailability of dabigatran pellets is 37% higher than that for capsules and the bioavailability increases to 75% when the capsule shell is removed; dabigatran capsules should not be tampered with in any way prior to administration. The Cmax is achieved by one hour following oral dosing, which is extended to two hours if accompanied by a high-fat meal. Dabigatran can be taken with or without food. Dabigatran pharmacokinetics are approximately linear over a range of 10-400 mg in healthy adults and adult patients and it has an accumulation factor of two in adult and pediatric patients.
Toxicity
No human studies involving pregnancy, labor and delivery, nursing, or pediatrics. Geriatric patients are at higher risk of adverse effects than younger patients but the risk to benefit ratio is generally still favourable for older patients. Patients with a creatinine clearance of 15-30mL/min should have their doses of dabigatran etexilate reduced, and no data is available for patients with a creatinine clearance below 15mL/min.
In animal studies, dabigatran increases the rates of dead offspring and causes uterine and vaginal bleeding close to birth. Dabigatran may or may not be excreted in breast milk so the risk and benefit of stopping the drug or stopping breast feeding must be considered.
Description
Dabigatran etexilate is an anticoagulant drug developed and marketed by Boehringer Ingelheim. The parent compound, dabigatran, is a potent thrombin blocker, but it cannot be administered orally. Adding the hydrophobic carbohexoxy group (“etexilate”) to the imine nitrogen created an orally absorbable prodrug.
Background
Dabigatran etexilate is an oral prodrug that is hydrolyzed to the competitive and reversible direct thrombin inhibitor dabigatran. Dabigatran etexilate may be used to decrease the risk of venous thromboembolic events in patients in whom anticoagulation therapy is indicated. In contrast to warfarin, because its anticoagulant effects are predictable, lab monitoring is not necessary. Dabigatran etexilate was approved by the FDA in 2010.
Properties of Dabigatran etexilate
| Melting point: | 128-129° |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.88±0.46(Predicted) |
| color | White to Pale Yellow |
| Stability: | Hygroscopic |
Safety information for Dabigatran etexilate
Computed Descriptors for Dabigatran etexilate
Dabigatran etexilate manufacturer
Clickchem Research LLP
SRINI PHARMACEUTICALS PVT LTD
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 DABIGATRAN ETEXILATE 35%, 40.0%, 43%, 50% W/W PELLETS 211915-06-9 98%View Details
DABIGATRAN ETEXILATE 35%, 40.0%, 43%, 50% W/W PELLETS 211915-06-9 98%View Details
211915-06-9 -
 211915-06-9 98%View Details
211915-06-9 98%View Details
211915-06-9 -
 Dabigatran etexilate 211915-06-9 98%View Details
Dabigatran etexilate 211915-06-9 98%View Details
211915-06-9 -
 Dabigatran etexilate 98% CAS 211915-06-9View Details
Dabigatran etexilate 98% CAS 211915-06-9View Details
211915-06-9 -
 Dabigatran etexilate 95% CAS 211915-06-9View Details
Dabigatran etexilate 95% CAS 211915-06-9View Details
211915-06-9 -
 Dabigatran etexilate 98% CAS 211915-06-9View Details
Dabigatran etexilate 98% CAS 211915-06-9View Details
211915-06-9 -
 Dabigatran Etexilate Powder APIView Details
Dabigatran Etexilate Powder APIView Details
211915-06-9 -
 99% Dabigatran Etexilate (working standard), Analytical GradeView Details
99% Dabigatran Etexilate (working standard), Analytical GradeView Details
211915-06-9
