PRODUCT INTRODUCTION
Description
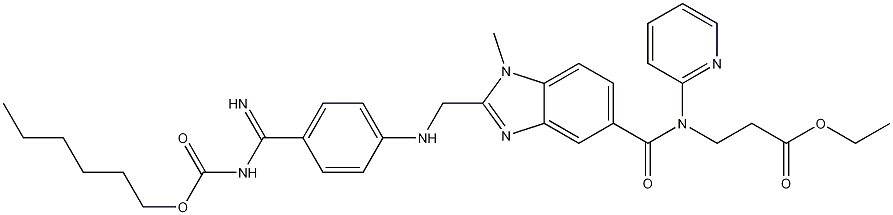
In March 2008, the European Commission granted marketing authorization for dabigatran etexilate (Pradaxa; Boehringer Ingelheim), a direct thrombin inhibitor, for the prevention of venous thromboembolic events in patients who have undergone total hip- or knee-replacement surgery.
RELATED SUPPLIERS
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Maharashtra
product:Dabigatran etexilate