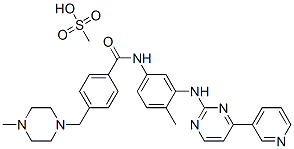
Imatinib mesylate
Synonym(s):Imatinib mesylate;Glivec;Imatinib methanesulfonate;4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate;4-[(4-Methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide mesylate
- CAS NO.:220127-57-1
- Empirical Formula: C30H35N7O4S
- Molecular Weight: 589.71
- MDL number: MFCD04307699
- EINECS: 606-892-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Imatinib mesylate?
Description
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. Imatinib mesylate is a c-Abl, PDGFR, and c-kit inhibitor that halts chronic myelogenous leukemia related growth. Imatinib is the first of a new class of anticancer drugs that are specifically designed to target the molecular pathways involved (oncogenic event) in the development of disease. The Brc-Abl oncoprotein is a constitutively active tyrosine kinase that causes CML.
Chemical properties
Off-White Solid
Originator
Novartis (Switzerland)
The Uses of Imatinib mesylate
A tyrosine kinase inhibitor. Highly specific for BCR-ABL, the enzyme associated with chronic myelogenous leukemia (CML) and certain forms of acute lymphoblastic leukemia (ALL)
The Uses of Imatinib mesylate
Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib
The Uses of Imatinib mesylate
Imatinib Mesylate (STI571) is an orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively.
The Uses of Imatinib mesylate
echinocandin antifungal, active against infections with Aspergillus and Candida, inhibits cell wall synthesis
Definition
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours.
brand name
Gleevec, Glivec
General Description
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome.
Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease.
Biochem/physiol Actions
Imatinib mesylate is a tyrosine kinase inhibitor with antineoplastic activity. Imatinib is a potent inhibitor of the Bcr-Abl kinase encoded by the bcr-abl oncogene as well as receptor tyrosine kinases encoded by c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes. Imatinib mesylate inhibition of Bcr-Abl tyrosine kinase created by the Philadelphia chromosome abnormality found in CML decreases proliferation and enhances apoptosis in leukemias CML and ALL. Inhibition of c-kit tyrosine activity inhibits mast-cell and cellular proliferation in those diseases overexpressing c-kit such as gastrointestinal stromal tumor (GIST).
The Uses of Imatinib mesylate
Imatinib mesylate is a c-Abl, PDGFR, and c-kit inhibitor that halts chronic myelogenous leukemia related growth.
Bioactivity
Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib. Imatinib is a competitive inhibitor of this tyrosine kinase as well as Abl, Kit and the PDGFR kinases It binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates and consequently blocks the proliferation of the leukemic cells.
Adverse Effects
The drug is well tolerated, producing few side effects, classified as grade 1 nausea, muscle cramps, diarrhea, edema and vomiting.
Storage
+4°C
Synthesis
Quetiapine fumarate can be prepared by a four step sequence from a condensation of the 1-(3-pyridyl)ethanone with dimethyl formamide dimethylacetal, followed by successive cyclization with the methyl-nitrophenyl guanidine, hydrogenolysis and condensation with the benzoyl chloride of the methylpiperazine.
References
1) Buchdunger, et al.(1996) Inhibition of the Abl Protein-Tyrosine Kinase in Vitro and in Vivo by a 2-Phenylpyrimidine Derivative; Cancer Res. 56 100 2) Heinrich et al (2000) Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 96 925 3) Morioka et al. (2016) Effect of Collagen Type 1 or Human Fibronectin on Imatinib Cytotoxicity in Oral Squamous Cell Carcinoma; Pharmacology and Pharmacy, 7 255 [Focus Biomolecules Citation] 4) Hazekawa et al. (2017) Assessment of cytotoxicity of imatinib for oral squamous cell carcinoma by a real-time cell analysis system; E. J. Bio., 13 56 [ Focus Biomolecules Citation]
Properties of Imatinib mesylate
| Melting point: | 214-224°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | White solid |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 220127-57-1(CAS DataBase Reference) |
Safety information for Imatinib mesylate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Imatinib mesylate
| InChIKey | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
Imatinib mesylate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4-Chloromethyl-N-[4-methyl-3-[[4-(pyridin-3-yl)pyrimidin-2-yl]amino]phenyl]benzamide](https://img.chemicalbook.in/CAS/GIF/404844-11-7.gif)



You may like
-
 Imatinib Mesylate 99%View Details
Imatinib Mesylate 99%View Details -
 Imatinib Mesylate 99%View Details
Imatinib Mesylate 99%View Details -
 Imatinib methanesulfonate >98% (HPLC) CAS 220127-57-1View Details
Imatinib methanesulfonate >98% (HPLC) CAS 220127-57-1View Details
220127-57-1 -
 Imatinib mesylate CASView Details
Imatinib mesylate CASView Details -
 Imatinib Mesylate Powder APIView Details
Imatinib Mesylate Powder APIView Details
220127-57-1 -
 Imatinib Mesylate, for CommercialView Details
Imatinib Mesylate, for CommercialView Details
152459-95-5 -
 Imatinib Mesylate Ip IhsView Details
Imatinib Mesylate Ip IhsView Details
220127-57-1 -
 Chemland Ind Imatinib Mesylate CAS NO 152459-95-5View Details
Chemland Ind Imatinib Mesylate CAS NO 152459-95-5View Details
220127-57-1
