Panobinostat
- CAS NO.:404950-80-7
- Empirical Formula: C21H23N3O2
- Molecular Weight: 349.43
- MDL number: MFCD09833242
- EINECS: 803-814-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
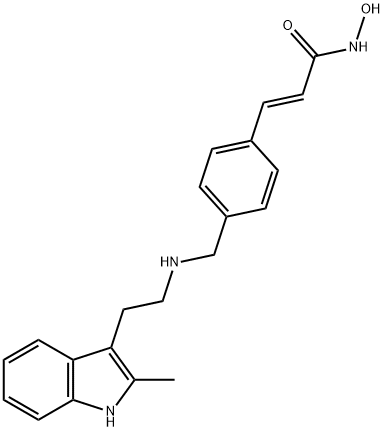
What is Panobinostat?
Absorption
After a 20 mg dose, panobinostat was quickly absorbed with a time to maximum absorption of 2 hours.
Toxicity
Farydak carries a Boxed Warning alerting patients and health care professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias and electrocardiogram (ECG) changes have occurred in patients receiving Farydak. Because of these risks, Farydak is being approved with a Risk Evaluation and Mitigation Strategy (REMS) consisting of a communication plan to inform health care professionals of these risks and how to minimize them.
Description
Panobinostat is a potent inhibitor of all histone deacetylases (HDACs), with Ki values ranging from 0.6 to 31 nM for HDAC1-
Chemical properties
Yellow Solid
The Uses of Panobinostat
The novel labelled histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells.
The Uses of Panobinostat
The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells.
Indications
Panobinostat is indicated in the treatment of multiple myeloma in combination with dexamethasone and bortezomib in patients who have received 2 previous treatment regimens including bortezomib and an immunomodulatory agent. This indication is approved by accelerated approval based on progression free survival as of February 23, 2015.
Background
Panobinostat is an oral deacetylace (DAC) inhibitor approved on February 23, 2015 by the FDA for the treatment of multiple myeloma. The approval was accelerated based on progression-free survival, therefore confirmatory trials by the sponsor to demonstrate clinical efficacy in multiple myeloma treatment are in progress of being conducted. Panobinostat is marketed by Novartis under the brand name Farydak. Panobinostat acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor) and it is the most potent DAC inhibiting agent available on the market.
What are the applications of Application
Panobinostat is a histone deacetylase (HDAC) inhibitor reported to inhibit human multiple myeloma cell lines.
Definition
ChEBI: A hydroxamic acid obtained by formal condensation of the carboxy group of (2E)-3-[4-({[2-(2-methylindol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enoic acid with the amino group of hydroxylamine. A histone deacetylase inhibitor used (as its la tate salt) in combination with bortezomib and dexamethasone for the treatment of multiple myeloma.
Clinical Use
Histone deacetylase (HDAC) inhibitor:
Treatment of relapsed and/or refractory multiple
myeloma
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with dextromethorphan possibly
increased risk of ventricular arrhythmias with
methadone - avoid.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone and possibly
disopyramide - avoid.
Antibacterials: increased risk of ventricular
arrhythmias with clarithromycin and moxifloxacin -
avoid; avoid with rifampicin.
Antidepressants: avoid with St John’s wort.
Antiepileptics: avoid with carbamazepine,
fosphenytoin, phenobarbital, phenytoin and
primidone.
Antifungals: concentration increased by ketoconazole
and possibly posaconazole and voriconazole - reduce
panobinostat dose; concentration possibly increased
by itraconazole - avoid.
Antimalarials: possibly increased risk of ventricular
arrhythmias with chloroquine - avoid.
Antipsychotics: avoid with pimozide.
Antivirals: concentration possibly increased
by ritonavir and saquinavir - reduce dose of
panobinostat.
Beta-blockers: possibly increased risk of ventricular
arrhythmias with sotalol - avoid.
Grapefruit juice: avoid concomitant use.
5HT3
antagonists: possibly increased risk of
ventricular arrhythmias with granisetron and
ondansetron - avoid with granisetron.
Metabolism
Panobinostat was extensively metabolized to 77 metabolites. Unchanged panobinostat recovered in urine and feces was 2% and 3%, respectively. Primary metabolic pathways of panobinostat are reduction, hydrolysis, oxidation, and glucuronidation processes. CYP and non-CYP enzymes were found to play significant role in metabolism, CYP2D6 and CYP2C19 playing minor roles.
Metabolism
Panobinostat is extensively metabolised, and a large
fraction of the dose is metabolised before reaching
the systemic circulation by reduction, hydrolysis,
oxidation and glucuronidation. Oxidative metabolism
of panobinostat played a less prominent role, with
approximately 40% of the dose eliminated by this
pathway. Cytochrome P450 3A4 (CYP3A4) is the main
oxidation enzyme, with potential minor involvement of
CYP2D6 and 2C19.
Panobinostat represented 6-9% of the drug-related
exposure in plasma. The parent substance is deemed to
be responsible for the overall pharmacological activity of
panobinostat.
After a single oral dose of [14C]-panobinostat in patients,
29-51% of administered radioactivity is excreted in the
urine and 44-77% in the faeces. Unchanged panobinostat
accounted for <2.5% of the dose in urine and <3.5% of
the dose in faeces.
Storage
Store at -20°C
References
1) Geng et al. (2006), Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer; Cancer Res., 66 11298 2) George et al. (2005), Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3; Blood, 105 1768 3) Maiso et al. (2006), The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance; Cancer Res., 66 5781
Properties of Panobinostat
| Melting point: | 114-117?C |
| Density | 1.241±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Soluble in DMSO (up to 100 mg/ml) or in Ethanol (up to 5 mg/ml with warming). |
| form | solid |
| pka | 8.71±0.23(Predicted) |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
Safety information for Panobinostat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Panobinostat
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![(1S,4Z,7S,10S,11E,20R)-4-ethylidene-7,20-dipropan-2-yl-9-oxa-15,16-dit hia-3,6,18,21-tetrazabicyclo[8.7.6]tricos-11-ene-2,5,8,19,22-pentone](https://img.chemicalbook.in/CAS/GIF/128517-07-7.gif)


You may like
-
 Panobinostat 95% CAS 404950-80-7View Details
Panobinostat 95% CAS 404950-80-7View Details
404950-80-7 -
 LBH-589 >95% CAS 404950-80-7View Details
LBH-589 >95% CAS 404950-80-7View Details
404950-80-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
