Rivaroxaban
- CAS NO.:366789-02-8
- Empirical Formula: C19H18ClN3O5S
- Molecular Weight: 435.88
- MDL number: MFCD11974010
- EINECS: 685-132-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
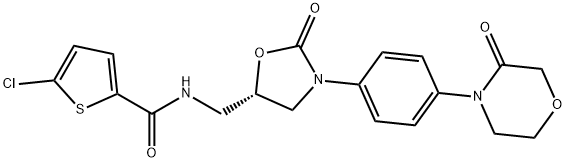
What is Rivaroxaban?
Toxicity
Excessive bleeding. Overdosages should be treated using activated charcoal and supportive measures such as mechanical compression and hemodynamic support. If bleeding is not controlled, the following procoagulants can be administered: activated prothrombin complex concentrate, prothrombin complex concentrate and recombinant factor VIIa. There is also a higher chance of post procedural hemorrhage compared to enoxaparin (1.55% vs. 1.39% respectively).
Description
Rivaroxaban is an orally active, direct inhibitor of Factor Xa (Ki = 0.4 nM), which is a crucial component of the intrinsic and extrinsic pathways of the blood coagulation cascade. It demonstrates >10,000-fold greater selectivity for Factor Xa compared to other related serine proteases (thrombin, trypsin, plasmin, FVIIa, FIXa, FXIa, urokinase, and activated protein C). In various animal arterial and venous thrombosis models, rivaroxaban is reported to inhibit thrombin formation without prolonging bleeding time and has been approved for clinical use as an anticoagulant in the prevention of stroke and the treatment of venous thromboembolisms.
Chemical properties
White Solid.
Rivaroxaban has limited pH-independent solubility in aqueous medium (5–7 mg/L; pH 1–9), but is, for instance, slightly soluble in polyethylene glycol 400 (2,431 mg/L). Using a validated Caco-2 cell assay, the apparent permeability values of the rivaroxaban molecule at concentrations of 1–100 μM were approximately 8 × 10?6 cm/s. With a log P value (octanol/water partition) coefficient of 1.5, rivaroxaban exhibits moderate lipophilicity, reflected in its low-to-moderate affinity to peripheral tissues[1].
IUPAC: 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide
Originator
Bayer (Germany)
The Uses of Rivaroxaban
Rivaroxaban is a novel antithrombotic agent. It is a novel, oral, selective direct inhibitor of factor Xa developed by Bayer Healthcare. It has been approved by the EMEA and FDA for the prevention ofvenous thromboembolism in adult patients after total hip replacement or total kneereplacement surgery.
Background
Rivaroxaban is an anticoagulant and the first orally active direct factor Xa inhibitor. Unlike warfarin, routine lab monitoring of INR is not necessary. However there is no antidote available in the event of a major bleed. Only the 10 mg tablet can be taken without regard to food. The 15 mg and 20 mg tablet should be taken with food. FDA approved on July 1, 2011.
Indications
Rivaroxaban is indicated for the prevention of venous thromboembolic events (VTE) in patients who have undergone total hips replacements and total knee replacement surgery; prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE); to reduce risk of recurrent DVT and/or PE. Rivaroxaban is also indicated, in combination with aspirin, for reducing the risk of major cardiovascular events in patients with chronic coronary artery disease or peripheral artery disease. Its use is also not recommended in those with severe renal impairment (<30mL/min).
Rivaroxaban is also indicated for the treatment and prevention of VTE in pediatric patients (from birth to 18 years of age) and for thromboprophylaxis in pediatric patients ≥2 years old with congenital heart disease following the Fontan procedure.
Definition
ChEBI: Rivaroxaban is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of 5-chlorothiophene-2-carboxylic acid with the amino group of 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one. An anticoagulant used for prophylaxis of venous thromboembolism in patients with knee or hip replacement surgery. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a member of thiophenes, an organochlorine compound, an oxazolidinone, a member of morpholines, a lactam, an aromatic amide and a monocarboxylic acid amide.
brand name
Xarelto
Pharmacokinetics
Rivaroxaban is an oral anticoagulant drug that is rapidly absorbed and reaches peak plasma concentrations within 2-4 hours of oral administration.The bioavailability of a 10 mg dose is greater than 80%. However, if a 15-20 mg dose is taken on an empty stomach, the bioavailability is lower and should be taken with food. Approximately two-thirds of the dose of rivaroxaban is metabolised. It is metabolised by CYP3A4, CYP3A5, CYP2J2 and CYP-dependent mechanisms.
Side Effects
Common side effects of Rivaroxaban include: back pain, headache, bleeding gums, coughing up blood, blood in the stool, bowel or bladder dysfunction, burning, itching, numbness, tingling, "pins and needles" or prickling sensation; difficulty breathing or swallowing, dizziness, increased menstrual flow or vaginal bleeding, prolonged bleeding from cuts, weakness in the legs, urine that is red or dark brown; vomiting blood or a substance that looks like coffee grounds. Occasionally fainting, arm or leg pain, wound discharge. Rare side effects include a burning sensation when passing urine, difficulty or pain in passing urine.
Pharmacokinetics
Rivaroxaban was absorbed rapidly with maximum plasma concentrations (Cmax) being reached 2–4 h after a single dose (1.25–80 mg) and multiple doses (up to 30 mg twice daily). Rivaroxaban did not accumulate to a relevant extent after multiple dosing. Data from phase I studies in healthy subjects showed that absorption is almost complete (oral bioavailability approached 80–100 %) for the 10 mg tablet dose, irrespective of fasting or fed conditions[3]. When administered orally with food, AUC and Cmax values were similar for whole and crushed rivaroxaban 20 mg tablets, whereas a crushed tablet suspended in water, administered using a nasogastric tube and followed by a liquid meal, gave similar AUC values but an 18 % reduction in Cmax.
Clinical Use
Factor Xa inhibitor:
Prevention of venous thromboembolism in adult
patients undergoing elective hip or knee replacement
surgery
Treatment of DVT or PE
Prophylaxis of stroke in AF
Prophylaxis of atherothrombotic events in ACS
Side Effects
Regarding safety, there was no statistical difference in the incidence of major postoperative bleeding between any of the rivaroxaban dose groups and enoxaparin although there did appear to be a dose dependency in the rivaroxaban set. In addition to bleeding and subsequent posthemorrhagic anemia, presenting as weakness, paleness, asthenia, dizziness, headache, or unexplained swelling, other common adverse events included nausea, increased GGT, and an increase in transglutaminase. Owing to its mechanism of action, there is a bleeding risk, so the drug is contraindicated in patients with clinically active bleeding. Rivaroxaban is also contraindicated in pregnant and breast-feeding women and in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk.
Synthesis
To date, several methods have been reported for the synthesis of rivaroxaban. Most of them share the use of 5-S-hydroxymethyl or 5-S-aminomethyl oxazolidinones (2and 3 respectively) as key intermediates. Condensation of 3-morpholinone with 4-fluoronitrobenzene followed by catalytic hydrogenation provides N-(p-aminophenyl)morpholinone for subsequent reaction with (S)-2-(phthalimidomethyl)oxirane. With establishment of the aminoalcohol adduct, cyclization with 1,1′-carbonyldiimidazole generates the central oxazolidinone. Deprotection and acylation with 5-chlorothiophene-2-carbonyl chloride affords rivaroxaban.
An Improved Synthesis of Rivaroxaban
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of haemorrhage with IV
diclofenac and ketorolac - avoid.
Antibacterials: concentration reduced by rifampicin.
Anticoagulants: increased risk of haemorrhage with
other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by
St John's wort.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: concentration increased by ketoconazole
- avoid; avoid with itraconazole, posaconazole and
voriconazole.
Antivirals: avoid with atazanavir, darunavir,
fosamprenavir, indinavir, lopinavir, saquinavir and
tipranavir; avoid with lopinavir; concentration
increased by ritonavir - avoid.
Cobicistat: possibly enhanced effect with cobicistat
- avoid.
Mechanism of action
Rivaroxaban--the first oral, direct Factor Xa inhibitor--is a small-molecule oxazolidinone derivative that binds directly and reversibly to Factor Xa via the S1 and S4 pockets. Rivaroxaban competitively inhibits Factor Xa and is more than 10,000-fold more selective for Factor Xa than other related serine proteases, and it does not require cofactors (such as antithrombin) to exert its anticoagulant effect. Unlike indirect Factor Xa inhibitors, rivaroxaban inhibits both free and clot-bound Factor Xa, as well as prothrombinase activity, thereby prolonging clotting times.
Metabolism
Metabolised by the cytochrome P450 isoenzymes CYP3A4 and CYP2J2 and by other mechanisms. About two-thirds of an oral dose is metabolised, with the metabolites excreted equally in the urine and faeces; the remaining third is excreted unchanged in the urine, mainly by active renal secretion.
Mode of action
Rivaroxaban is a direct, specific Factor Xa inhibitor. In vitro kinetic studies showed that the inhibition of human Factor Xa by rivaroxaban was competitive [inhibition constant (K i) 0.4 ± 0.02 nM] with 10,000-fold greater selectivity than for other serine proteases—it does not inhibit related serine proteases at concentrations up to 20 μM. This compound potently inhibited prothrombinase activity [inhibitory concentration 50 % (IC50) 2.1 ± 0.4 nM], and clot-associated Factor Xa activity (IC50 75 nM). In human plasma, rivaroxaban inhibited endogenous Factor Xa activity in a concentration-dependent manner with an IC50 of 21 ± 1 nM [19]. In an ex vivo study, rivaroxaban at a single dose of 5 mg and 30 mg reduced collagen-induced endogenous thrombin potential in human plasma by approximately 80 and 90 %, respectively, and tissue factor-induced endogenous thrombin potential by approximately 40 and 65 %, respectively. In contrast to indirect Factor Xa inhibitors, rivaroxaban does not require any cofactor to exert its anticoagulant effect. In human plasma, rivaroxaban concentration-dependently inhibited thrombin generation and, thus, the amplification processes of coagulation. Thrombin generation was almost completely inhibited at therapeutically relevant concentrations (80–100 nM) of rivaroxaban. In addition, rivaroxaban increased the permeability and degradability of the whole blood clot, by decreasing thrombin generation. However, rivaroxaban does not inhibit the activity of pre-existing thrombin molecules[1-2].
References
[1] Mueck, W., et al. "Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban."Springer Open Choice 53(2014):1-16.
[2] Perzborn, E., et al. "In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor." Journal of Thrombosis and Haemostasis 3.3(2005):514-521.
[3] Kubitza, Dagmar , et al. "Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects." European Journal of Clinical Pharmacology 61.12(2005):873-80.
Properties of Rivaroxaban
| Melting point: | 228-229°C |
| Boiling point: | 732.6±60.0 °C(Predicted) |
| Density | 1.460±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥13.9 mg/mL in DMSO with gentle warming |
| form | solid |
| pka | 13.36±0.46(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 |
| appearance | White to Light yellow powder to crystal |
Safety information for Rivaroxaban
Computed Descriptors for Rivaroxaban
| InChIKey | KGFYHTZWPPHNLQ-AWEZNQCLSA-N |
| SMILES | C1(C(NC[C@@H]2OC(=O)N(C3=CC=C(N4CCOCC4=O)C=C3)C2)=O)SC(Cl)=CC=1 |
Rivaroxaban manufacturer
KP INNOVATIVE
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Rivaroxaban 99%View Details
Rivaroxaban 99%View Details -
 Rivaroxaban 98%View Details
Rivaroxaban 98%View Details -
 Rivaroxaban 98%View Details
Rivaroxaban 98%View Details -
 Rivaroxaban 99%View Details
Rivaroxaban 99%View Details -
 Rivaroxaban 99%View Details
Rivaroxaban 99%View Details -
 Rivaroxaban 98%View Details
Rivaroxaban 98%View Details -
 Rivaroxaban 98%View Details
Rivaroxaban 98%View Details -
 Rivaroxaban 98%View Details
Rivaroxaban 98%View Details
