Chromium(VI) oxide
Synonym(s):Chromic anhydride;Chromic anhydride, Chromium trioxide;Chromium(VI) oxide
- CAS NO.:1333-82-0
- Empirical Formula: CrO3
- Molecular Weight: 99.99
- MDL number: MFCD00010952
- EINECS: 215-607-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
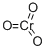
What is Chromium(VI) oxide?
Description
Chromium hydroxide (Cr2O(OH)4) is a bright bluish-green pigment prepared by the calcinations of bichromate with boric acid at 500°C. The mass during cooling is hydrolyzed with water, which yields the hydrate.
Description
Chromium trioxide (CrO3) is a dark red/orange solid that acts as a strong oxidizing agent. In organic chemistry it is most often associated with Jones oxidation.
Chemical properties
Chromium trioxide is a dark-red crystalline substance. It is odorless
Physical properties
Dark-red crystals, flakes or granular powder; bipyramidal prismatic system; density 2.70 g/cm3; melts at 197°C; decomposes on further heating; highly soluble in water, 61.7 g and 67 g/100 mL at 0°C and 100°C, respectively; soluble in sulfuric and nitric acids.
The Uses of Chromium(VI) oxide
Chromium(VI) oxide is widely used in chromium electroplating, and as protective coatings in corrosion/oxidation resistance of metals. It is also employed in CDs and DVDs. It is useful as an oxidant in Jones oxidation in acetic acid or acetone. It is used in the oxidation of primary alcohols into carboxylic acids, and secondary alcohols into ketones. With phosphoric acid, it is used as a stripping agent for anodic coatings of all types and in the production of syntheric rubies.
The Uses of Chromium(VI) oxide

To an orange, homogeneous solution of CrO3 (12.4 g, 0.123 mol) in H2O (88.4 mL) at 0 C was added H2SO4 (10.8 mL) dropwise via addition funnel over 30 min, with stirring. The addition funnel was rinsed with H2O (1 mL) to give a 1.23 M solution of Jones Reagent. To a solution of the SM (5.24 g, 21.6 mmol) in acetone (75 mL) at RT (immersed in H2O bath) was added Jones Reagent (43.8 mL, 53.9 mmol) via addition funnel over 90 min. The dark reaction mixture was stirred at RT overnight. By HPLC, the reaction was 93% complete. Additional Jones Reagent (18 mL, 1.0 equiv) was added. After stirring another 6.5 h, HPLC indicated 97% completion. Isopropanol (6 mL) was added, and the mixture stirred for 90 min, resulting in a dark green precipitate. The mixture was diluted with ether (600 mL) and washed with 2% aq NaHSO3 (5 x 100 mL). The layers were separated and the aq layer was back-extracted with ether (2 x 100 mL). The combined organics were washed with H2O (100 mL), brine (100 mL), and dried (Na2SO4). The aq layer was back-extacted with ether (100 mL), and the resulting org layer was combined with previous organics. The organics were concentrated to provide the pdt as an off-white solid. The pdt was dissolved in DCM (200 mL), washed with 2% aq NaHSO3, brine, dried (Na2SO4), and concentrated to provide the pdt (96% purity) as a pale yellow solid [3.84 g, 69.3%]. Additional pdt remained in the NaHSO3 aq layer. The aq layer was saturated with NaCl, the pH adjusted to ~3.5, and extracted with ether (3 x 100 mL). The organics were dried (Na2SO4) and concentrated to provide the product (99% purity) as a white solid [1.12 g, 20.2%]. Total yield: [4.96 g, 89.5%]
What are the applications of Application
Chromium(VI) oxide is a reagent used for oxidation and in catalysis
Definition
ChEBI: A chromium oxide composed of a single chromium bound (oxidation state +6) to three oxygens; the acidic anhydride of chromic acid.
Preparation
Chromium(VI) oxide is prepared by heating sodium dichromate dihydrate with a slight excess of sulfuric acid in a steel tank or cast iron container: Na2Cr2O7 + 2H2SO4 → 2CrO3 + 2NaHSO4 + H2O The temperature of the mixture is kept above the melting point of chromium(VI) oxide to evaporate water and separate the top layer of sodium bisulfate from the molten chromium(VI) oxide at the bottom. Temperature control and duration of heating is very crucial in the process. Temperatures over 197°C (melting point), or allowing the molten mass to stand for a longer time, may result in decomposition of the product.
Definition
chromium trioxide: A redcompound, CrO3; rhombic; r.d. 2.70;m.p. 196°C. It can be made by carefuladdition of concentrated sulphuricacid to an ice-cooled concentratedaqueous solution of sodium dichromatewith stirring. The mixture isthen filtered through sintered glass,washed with nitric acid, then dried at120°C in a desiccator.
Chromium(VI) oxide is an extremelypowerful oxidizing agent,especially to organic matter; it immediatelyinflames ethanol. It is anacidic oxide and dissolves in water toform ‘chromic acid’, a powerful oxidizingagent and cleansing fluid forglassware. At 400°C, chromium(VI)oxide loses oxygen to givechromium(III) oxide.
General Description
Chromium(VI) oxide (CrO3) is a hexavalent chromium that is majorly used as an oxidizing agent. It oxidizes the C-H bonds in the aromatic rings to form benzoic acid from alkyl benzene. It can be prepared by adding concentrated sulphuric acid in potassium dichromate.
Air & Water Reactions
Deliquescent. Water soluble, giving acidic solutions.
Reactivity Profile
CHROMIUM TRIOXIDE is a powerful oxidizing agent. Can react violently upon contact with reducing reagents, including organic matter, leading to ignition or explosion. Dangerously reactive with acetone, alcohols, alkali metals (sodium, potassium), ammonia, arsenic, dimethylformamide, hydrogen sulfide, phosphorus, peroxyformic acid, pyridine, selenium, sulfur, and many other chemicals [Sax, 9th ed., 1996, p. 852]. Noncombustible but can accelerate the burning of combustible materials. Sufficient heat may be generated from the reaction with combustible materials to ignite the mass. Aqueous solutions corrode many metals rapidly. Often mixed with sulfuric acid to make "cleaning solution" for glass. Used cleaning solution in closed bottles may explode due to the build up of gaseous carbon dioxide arising from oxidation of organic impurities [Bryson, W. R., Chem. Brit., 1975, 11, p. 377].
Health Hazard
Chromium(VI) oxide and other chromium(VI) salts are moderately toxic substances by ingestion; 1 to 15 g may be a fatal dose in humans. Ingestion of nonlethal doses of these compounds can cause stomach, liver, and kidney damage; symptoms may include clammy, cyanotic skin, sore throat, gastric burning, vomiting, and diarrhea. Chromic acid is irritating to the skin, and prolonged contact can cause ulceration. Inhalation of chromate dust or chromic acid mist can result in severe irritation of the nose, throat, bronchial tubes, and lungs and may cause coughing, labored breathing, and swelling of the larynx. Eye contact with chromium trioxide and its solutions can cause severe burns and possible loss of vision.
Occupational exposure to chromium(VI) compounds has been related to an increased risk of lung cancer. Several hexavalent compounds of chromium, including chromium trioxide, are listed in IARC Group 1 ("carcinogenic to humans") and are classified as "select carcinogens" under the criteria of the OSHA Laboratory Standard. Long-term exposure to chromium trioxide or chromium(VI) salts may cause ulceration of the respiratory system and skin. Exposure to chromium trioxide by inhalation or skin contact may lead to sensitization. Chromium trioxide has exhibited teratogenic activity in animal tests.
Fire Hazard
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Chromium(VI) oxide is not combustible but is a strong oxidizing agent and can accelerate the burning rate of combustible materials. Contact with easily oxidized organic or other combustible materials (including paper and oil) may result in ignition, violent combustion, or explosion. The use of dry chemical, carbon dioxide, Halon, or water spray extinguishers is recommended for fires involving chromium (VI) compounds.
Potential Exposure
Chromium trioxide is used in plating and metal treatment, as a corrosion inhibitor; and as an oxidant; in aluminum anodizing, dye; ink, and paint manufacturing, tanning, engraving; and photography.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting.
storage
Chromium trioxide should be handled in a fume hood to avoid the inhalation of dust, and impermeable gloves should be worn at all times to prevent skin contact. The practice of using chromate solutions to clean glassware should be avoided. Chromium trioxide should be stored in areas separated from readily oxidized materials.
Shipping
UN1463 (anhydrous), Chromium trioxide, anhydrous, Chromium trioxide, anhydrous, Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, 8-Corrosive material. UN1755 (solution), Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
It separates when potassium or sodium dichromate are dissolved in conc H2SO4. Dry it in a vacuum desiccator over NaOH pellets. It is a hygroscopic, powerful oxidant and can ignite with organic compounds. It is a skin and pulmonary IRRITANT. [Keyes et al. Industrial Chemicals (Lowenheim & Moran eds.) 4th edn J. Wiley pp 270-274 1975.] CANCER SUSPECT.
Properties and Applications
|
TEST ITEM |
STANDARD |
|
Appearance |
Dark red flake |
|
DENSITY |
2.7 |
|
Melt Point |
197°C |
|
Chromic anhydride(CrO3) |
99.6 % min |
|
Sulfate (SO4) |
0.10% max |
|
Water insolubles |
0.03% max |
|
Sodium (Na) |
- |
|
Turbidity |
15% max |
Incompatibilities
Chromium trioxide is a strong oxidizer. The solution in water is a strong acid. Reacts violently with bases and is corrosive. Contact with reducing agents; fuels, organic chemicals, flammable and combustible materials, causing fire and explosion hazard. This chemical decomposes above 250C to chromic oxide and oxygen with increased fire hazard. Attacks metals in the presence of moisture.
Waste Disposal
Reduce to Cr(III). If material cannot be recovered and recycled, dispose of sludge in a chemical waste landfill.
Properties of Chromium(VI) oxide
| Melting point: | 196 °C (dec.)(lit.) |
| Boiling point: | 330 °C |
| Density | 2.7 |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | 250°C |
| storage temp. | Store below +30°C. |
| solubility | 1.667g/l |
| form | macroporous |
| appearance | Red solid |
| pka | -0.61[at 20 ℃] |
| Specific Gravity | 2.7 |
| color | Reddish-violet |
| PH | <1 (100g/l, H2O, 20℃) |
| Water Solubility | Highly soluble |
| FreezingPoint | 170~172℃ |
| Sensitive | Hygroscopic |
| Merck | 14,2235 |
| Exposure limits | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| Stability: | Stable. Strong oxidizer. Reacts with most organic material in a violent and often explosive fashion. Moisture sensitive. |
| CAS DataBase Reference | 1333-82-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Chromium trioxide(1333-82-0) |
| EPA Substance Registry System | Chromium(VI) trioxide (1333-82-0) |
Safety information for Chromium(VI) oxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chromium(VI) oxide
| InChIKey | WGLPBDUCMAPZCE-UHFFFAOYSA-N |
Chromium(VI) oxide manufacturer
ARRAKIS INDUSTRIES LLP
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Chromium(VI) oxide 98%View Details
Chromium(VI) oxide 98%View Details -
 Chromium(VI) oxide 99%View Details
Chromium(VI) oxide 99%View Details -
 Chromium(VI) oxide CAS 1333-82-0View Details
Chromium(VI) oxide CAS 1333-82-0View Details
1333-82-0 -
 Chromium(VI) oxide CAS 1333-82-0View Details
Chromium(VI) oxide CAS 1333-82-0View Details
1333-82-0 -
 Chromium(VI) oxide CAS 1333-82-0View Details
Chromium(VI) oxide CAS 1333-82-0View Details
1333-82-0 -
 Chromium(VI) oxide, For analysis ACS CAS 1333-82-0View Details
Chromium(VI) oxide, For analysis ACS CAS 1333-82-0View Details
1333-82-0 -
 Chromium oxide, For analysis ACS CAS 1333-82-0View Details
Chromium oxide, For analysis ACS CAS 1333-82-0View Details
1333-82-0 -
 Chromium(VI) oxide CAS 1333-82-0View Details
Chromium(VI) oxide CAS 1333-82-0View Details
1333-82-0
