chromium dioxide
Synonym(s):Chromium dioxide;Chromium dioxide (CrO2 );Chromium oxide;Chromium(IV) oxide
- CAS NO.:12018-01-8
- Empirical Formula: CrO2
- Molecular Weight: 83.99
- MDL number: MFCD00049888
- EINECS: 234-630-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
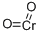
What is chromium dioxide?
Chemical properties
dark brown or black powder(s); tetr; used in magnetic tapes [KIR78]; ferromagnetic; rutile structure [MER06]
Physical properties
Chromium dioxide [12018-01-8], chromium(IV)oxide, CrO2, is a ferromagnetic material with a specific saturation magnetization Ms/? of 132 A m2 kg?1 at 0 K, corresponding to the spin of two unpaired electrons per Cr4+ ion. TheMs/n value of CrO2 at room temperature is ca. 100 A m2 kg?1; CrO2 magnetic pigments reach values of 77–92 A m2 kg?1. The material crystallizes with a tetragonal rutile lattice in the form of small needles, which have the desired magnetic shape anisotropy. The morphology of the particles can be varied with several dopants, particularly antimony and tellurium. The coercive field strength can be controlled between ca. 30 and 75 kA m?1 (in addition to shape) by doping with transition metal ions, which modify the magneto-crystalline anisotropy of the material; the Fe3+ ion being industrially important.
The Uses of chromium dioxide
Chromium dioxide is used exclusively for magnetic recording media. It may also be used in combination with cobalt-modified iron oxides in the production of magnetic recording media. The world consumption of CrO2 is decreasing continuously as compact disc and digital videodisc are taking over the audio and video market. The sole producer is DuPont.
In the course of the development of pigments for magnetic information storage, CrO2 was the first widely used pigment material that gave a higher recording density than ã-Fe2O3. In the field of audio recording this led to the IEC II standard or “chrome position”.
The Uses of chromium dioxide
In magnetic recording tapes; as catalyst.
The Uses of chromium dioxide
Facilitates high yield aromatization of Hantzsch 1,4-dihydropyridines to the corresponding pyridines.
Production Methods
The conversion of an intimate mixture of Cr(III) and Cr(VI) compounds into CrO2 under hydrothermal conditions has been developed into an industrial process in autoclaves at ca. 350 °C and 300 bar.
Pure CrO2 slowly disproportionates in the presence of water. The CrO2 crystal surface of commercial pigments is therefore topotactically converted to â-CrOOH, which serves as a protection layer. In the absence of moisture, CrO2 is stable up to ca. 400 °C, above this temperature it decomposes to form Cr2O3 and oxygen.
Properties of chromium dioxide
| Melting point: | >375 °C (dec.)(lit.) |
| Boiling point: | decomposes to Cr2O3℃ [KIR78] |
| Density | 4.85 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O; soluble in acid solutions |
| form | brown-black tetragonal powder |
| color | brown-black tetragonal
powder |
| Water Solubility | insoluble H2O, soluble acids giving Cr+++and dichromate [KIR78] |
| Merck | 13,2254 |
| CAS DataBase Reference | 12018-01-8 |
| EPA Substance Registry System | Chromium oxide (CrO2) (12018-01-8) |
Safety information for chromium dioxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for chromium dioxide
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
