Cadmium nitrate
Synonym(s):Cadmium nitrate solution
- CAS NO.:10325-94-7
- Empirical Formula: CdN2O6
- Molecular Weight: 236.42
- MDL number: MFCD00010915
- EINECS: 233-710-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
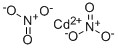
What is Cadmium nitrate?
Chemical properties
White, amorphous pieces or hygro- scopic needles. Soluble in water, ammonia, and alco- hol.
Physical properties
White crystal or amorphous powder; hygroscopic; density 3.60 g/cm3; melts at 350°C; very soluble in water, also soluble in alcohols.
The Uses of Cadmium nitrate
Cadmium salts, photographic emulsions, col- oring glass and porcelain, laboratory reagent, cad- mium salts.
Definition
ChEBI: An inorganic nitrate salt having Cd(2+) as the counterion.
Preparation
reparation Cadmium nitrate is prepared by dissolving cadmium metal or its oxide, hydroxide, or carbonate, in nitric acid followed by crystallization:
CdO + 2HNO3 → Cd(NO3)2 + H2O.
General Description
Odorless white solid. Sinks in water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109].
Hazard
Dangerous fire and explosion hazard.
Health Hazard
Inhalation of fumes can produce coughing, chest constriction, headache, nausea, vomiting, pneumonitis. Chronic poisoning is characterized by emphysema and kidney injury. Ingestion causes gastrointestinal disturbance and severe toxic symptoms; both kidney and liver injuries may occur. Contact with eyes causes irritation.
Safety Profile
Confirmed human carcinogen. Poison by ingestion and possibly other routes. Moderately toxic by inhalation. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cd and NOx. See also CADMIUM COMPOUNDS and NITRATES.
Properties of Cadmium nitrate
| Melting point: | 59.4℃ |
| Boiling point: | 132℃ |
| Density | 2.455(17/4℃) |
| solubility | soluble in ethanol |
| form | white cubic crystals |
| color | white cubic crystals, crystalline; hygroscopic |
| PH | ≥3 |
| Water Solubility | g/100g solution H2O: 55.1 (0.6°C), 61.3 (25°C), 87.2 (100°C); solid phase, Cd(NO3)2 · 4H2O (0°C, 25°C), Cd(NO3)2 (100°C) [KRU93]; soluble alcohol, ammonia [HAW93] |
| Merck | 13,1622 |
| BRN | 8137359 |
| CAS DataBase Reference | 10325-94-7(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium nitrate (10325-94-7) |
Safety information for Cadmium nitrate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity H350:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cadmium nitrate
Cadmium nitrate manufacturer
Kronox Lab Sciences Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Cadmium nitrate 98%View Details
Cadmium nitrate 98%View Details -
 Cadmium Standard for AAS CASView Details
Cadmium Standard for AAS CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8