Potassium nitrate
Synonym(s):Potassium nitrate;Sodium nitrate;Nitrate of Potash;Nitric acid potassium salt;Chile salpeter
- CAS NO.:7757-79-1
- Empirical Formula: KNO3
- Molecular Weight: 101.1032
- MDL number: MFCD00011409
- EINECS: 231-818-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:42
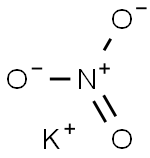
What is Potassium nitrate?
Absorption
It is established that nitrate is quickly and almost entirely absorbed from the proximal and small intestine subsequent to ingestion in most animals, with little if any absorption from the stomach and lower intestine .
The vast majority of intestinal K+ absorption occurs in the small intestine; the contribution of the normal colon to net K+ absorption and secretion is trivial .
Toxicity
Acute oral toxicity (LD50): 1901 mg/kg in rabbits and 3750 mg/kg in rats .
The primary acute toxic effect of nitrates is the development of methemoglobinemia, a condition in which greater than 10% of the hemoglobin in the body is transformed into methemoglobin. When this conversion exceeds 70% the condition may result in death .
The potassium ion by itself possesses very little toxicity; the toxicity of the salts is associated with the anion. Potassium nitrate is rapidly absorbed from the upper gastrointestinal tract and is excreted mostly as the unchanged drug . This excludes a small percentage of the ingested dose that is reduced by the microbial action of the gut to nitrite. Nitrites convert the hemoglobin in red blood cells into methemoglobin . In male rats given potassium nitrate, intestinal absorption was affected .
Adverse increased potassium intake included changes in blood lipids, triglyceride, decreased high-density lipoprotein [HDL] cholesterol), changes in renal function, and increases in catecholamine levels. The decrease in blood volume caused by increased potassium activates the sympathetic nervous system, resulting in the release of adrenaline and noradrenaline. Decreases in blood volume may also contribute to the observed changes in blood lipid concentrations .
Death and severe effects of nitrate ingestion are generally associated with doses of the drugs above 10g NO3-. Doses ranging from 2-9 g NO3- have been reported to cause methemoglobinemia. These values correspond to 33 - 150 mg NO3-/kg
Potassium nitrate was shown to cause low to moderate acute toxicity. Repeated dose toxicity was investigated in rats given oral doses in the range 10-100 mg/kg per day for 4 months; bronchopneumonia, local hemorrhages, and other circulatory disorders were observed in treated animals. Cattle were given oral doses of 345-450 mg/kg daily (expressed as nitrate) for several months; blood phosphate and magnesium were decreased and blood calcium, urinary magnesium, urea and milk urea were increased .
Description
Potassium nitrate is a solid, colorless, crystalline ionic compound that exists as the mineral niter.Potassium nitrate is also known as saltpeter. The name saltpeter comes from the Latin sal petrae, meaning salt of stone or salt of Petra. he term saltpeter or Chilean saltpeter is also used for sodium nitrate, NaNO3.
Chemical properties
Potassium nitrate is an odorless, flammable, water-soluble, white or colorless crystals with saline taste that melt at 337°C. Used in pyrotechnics, explosives, and matches, as a fertilizer, and as an analytical reagent.
Physical properties
Colorless transparent crystals or white granular or crystalline powder;rhombohedral structure; density 2.11 g/cm3at 20°C; melts at 334°C; decomposes at 400°C evolving oxygen; soluble in cold water, 13.3 g/100mL at 0°C;highly soluble in boiling water, 247 g/100mL at 100°C; lowers the temperature of water on dissolution; very slightly soluble in ethanol; soluble in glycerol and liquid ammonia.
History
Saltpeter’s most prominent use in human history is as the principal ingredient in gunpowder.The potassium nitrate used in gunpowder was originally obtained from natural mineral deposits of niter. Small quantities formed as efflorescence deposits on damp stone walls were identified as early as 2000 b.c.e. in Sumerian writings. As the use of black powder expanded with the development of weapons, the demand for saltpeter exceeded supply. This was exacerbated during times of war. To meet the demand for saltpeter to produce black powder, a saltpeter industry developed that followed prescribed methods to produce large quantities of saltpeter. The method depended on processing dirt obtained from areas where nitrates would naturally form. These were areas in which animal waste had accumulated such as the dirt floors of barns, stables, herding pens, caves, or cellars. The ammonia compounds in the urine and fecal wastes in these areas underwent nitrifi cation to produce nitrates, which combined with potassium in the soil to form saltpeter.
The Uses of Potassium nitrate
Although the most prominent use of saltpeter is for the production of black powder,potassium nitrate is also used as fertilizer. In the first half of the 17th century, JohannRudolf Glauber (1604–1668) obtained saltpeter from animal pens and discovered its useto promote plant growth. Glauber included saltpeter with other nutrients in fertilizer mixtures. Glauber’s work was one of the first to indicate the importance of nutrient cyclingin plant nutrition.
The Uses of Potassium nitrate
In fireworks, fluxes, pickling meats; production of nitric acid; manufacture of glass, matches, gunpowder; freezing mixtures. Agricultural fertilizer. Preservative in foods. In dentrifices to reduce tooth hypersensitivity.
The Uses of Potassium nitrate
Potassium Nitrate is a preservative and color fixative in meats which exists as colorless prisms or white granules or powder. it has a solubility of 1 g in 3 ml of water at 25°c. see nitrate.
The Uses of Potassium nitrate
This natural substance is the product of the decomposition of lime and urine. The white granules or powder are soluble in water 1:3 but insoluble in alcohol. Potassium nitrate, also called saltpeter or nitre, was combined with sulfuric acid to nitrate cotton for the manufacture of collodion. It was also used with magnesium to make flash powder and added to ferrous sulfate developers to produced cool white tones in collodion positives.
Indications
For the relief of tooth sensitivity, and is also used as a pesticide, insecticide, as a food additive, and a rodenticide .
Background
Potassium nitrate is an inorganic salt with a chemical formula of KNO3. It is a natural source of nitrate and has been used as a constituent for several different purposes, including food preservatives, fertilizers, tree stump removal, rocket propellants, and fireworks. Potassium nitrate is a common active ingredient in toothpaste, exerting an anti-sensitivity action. It provides increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact .
In addition, potassium nitrate is used as a diuretic in pigs, cattle, and horses. It is administered orally doses up to 30 g per animal per day .
What are the applications of Application
Potassium nitrate is a naturally occurring mineral source of nitrogen
Definition
ChEBI: The inorganic nitrate salt of potassium.
Production Methods
Potassium nitrate may be produced by several methods. It is made commercially by reacting potassium chloride with nitric acid at high temperature.Nitrosyl chloride, a product obtained in the reaction, is converted into chlorine in this manufacturing process. Also, nitric acid is partly recycled in the process. The reactions are (Dancy, W.B. 1981. Potassium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd. ed. Pp. 939-42. New York: Wiley Interscience):
3KCl + 4HNO3 →3KNO3+ Cl2+ NOCl + 2H2O
2NOCl + 4HNO3→6NO2+ Cl2+ 2H2O
4NO2+ O2+ 2H2O →4HNO3
Potassium nitrate also can be prepared by mixing a hot saturated solution of potassium chloride and sodium nitrate. The reaction is:
K++ Clˉ+ Na++ NO3ˉ→NaCl↓+ K++ NO3ˉ
Sodium chloride is less soluble than KCl, NaNo3and KNo3. It separates out by crystallization. The remaining solution is cooled to ambient tempera-ture. Potassium nitrate crystallizes out.
brand name
Cholal modifico;Cholal simple;Dewitt's pills for backache and joint pain;Viridite k.
World Health Organization (WHO)
Potassium nitrate was formerly used as a diuretic. Its use for this purpose is now considered obsolete but it is still available in at least one country for the correction of potassium deficiency. It is aslo widely permitted at concentrations of the order of 5% in proprietary toothpastes. In some countries the drug has been banned due to a potential carcinogenic risk arising from the excessive use of nitrates and their transformation to nitrosamines.
General Description
A white to dirty gray crystalline solid. Water soluble. Noncombustible, but accelerates the burning of combustible materials. If large quantities are involved in fire or the combustible material is finely divided an explosion may result. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in solid propellants, explosives, fertilizers.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Potassium nitrate mixed with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Powdered antimony mixed with Potassium nitrate explodes when heated [Mellor 9:282 1946-47]. A mixture of antimony trisulfide and Potassium nitrate explodes at a red heat [Mellor 9:524. 1946-47]. Arsenic disulfide forms explosive mixtures when mixed with Potassium nitrate, [Mellor 9:270.1946-47]. A mixture of sodium acetate and Potassium nitrate may cause an explosion [Pieters 1957. p. 30]. A mixture of Potassium nitrate and sodium hypophosphite constitutes a powerful explosive [Mellor 8:881. 1946-47]. A mixture of powdered zirconium and Potassium nitrate explodes when heated above the melting point [Mellor 7:116. 1946-47].
Hazard
Dangerous fire and explosion risk when shocked or heated, or in contact with organic mate- rials, strong oxidizing agent.
Health Hazard
Exposure can cause mild irritation of eyes, nose and throat.
Flammability and Explosibility
Non flammable
Agricultural Uses
Potassium nitrate (KNO3) is a potassium salt of nitric acid, also known as saltpeter or nitrate of potash. It is a white crystalline salt which occurs naturally in nitre or saltpeter. It can be used as fertilizer for normal application and fertigation. Potassium (44% K2O) and nitrogen (13 %) are the constituents of NK fertilizers, which serve as a source of potassium, where extra chloride is not desired.
The agricultural grade of potassium nitrate is freeflowing and non-caking, with a particle size in the range of 1500 to 400 microns.
Potassium nitrate, which is slightly hygroscopic and granulated, can be spread on soil by trucks, fertilizer distributors or by aerial spraying. In a mixed fertilizer, a powdered grade of nitrate of potash does not cake. Potassium nitrate is made by the reaction of potassium chloride with nitric acid as: The nitrate of potash forms an easily breakable crust on top. It is chemically neutral and its nitrogen and potassium oxide ratio is roughly 1:3. It has been used successfully as a source of nitrogen and potassium for tobacco, tomato, potato, corn, citrus and carnations.
Industrial uses
Potassium nitrate is also called niter and saltpeter,although these usually refer to the nativemineral. A substance of the composition KNO3,it is used in explosives, for bluing steel, and infertilizers. A mixture of potassium nitrate andsodium nitrate is used for steel-tempering baths.The mixture melts at 250°C. Potassium nitrateis made by the action of potassium chloride onsodium nitrate. It occurs in colorless prismaticcrystals, or as a crystalline white powder. It hasa sharp saline taste and is soluble in water. Thespecific gravity is 2.1 and the melting point is337°C.
Potassium nitrate contains a large percentageof oxygen, which is readily given up andis well adapted for pyrotechnic compounds. Itgives a beautiful violet flame in burning. It isused in flares and in signal rockets.
Most enamels contain some oxidizing agentin the form of potassium or sodium nitrate.Only a small amount of nitrate is necessary; 2to 4% is sufficient to maintain oxidizing conditionsin most smelting operations.
In glazes it is sometimes used as a flux inplace of potassium oxide, but, owing to its costand solubility, very little of it is contained inglaze. Where conditions prevent the use of sufficientpotash feldspar, potassium oxide is introducedinto the mix, usually in the form of thenitrate in a frit.
Potassium nitrite is a solid of the compositionKNO2 used as a rust inhibitor, for theregeneration of heat-transfer salts, and for themanufacture of dyes.
Biochem/physiol Actions
Potassium nitrate serves as a source of nitrate for cell growth. It also induces the synthesis of lipid in alga Neochloris oleoabundans.
Pharmacokinetics
The potassium cation is an essential electrolyte that is important for the maintenance of intracellular osmotic pressure and for the maintenance of cell membrane potential, in particular, the potential of electrically excitable tissues . It is a regular component of the diet and is particularly abundant in fruit and vegetables. The recommended daily intake varies from 350-1275 mg in children to 1875 and 5625 mg in adults. In the United Kingdom, the recommended intake is 3.5 g/day for healthy adults . Potassium ions are believed to disturb the synapse between nerve cells, thus decreasing nerve excitation and the associated pain .
Potassium nitrates are ignitable fumigants also utilized as rodenticides and insecticides. They are added to other pesticide active ingredients (sulfur and carbon) and placed into fumigant gas cartridges, designed to be ignited and placed in pest-infested areas. The activated cartridge bombs release toxic gases which are lethal to select rodents, skunks, coyotes, and wasps .
Potassium ions have demonstrated in animal studies to act directly on the nerves and to reduce sensory activity . Tooth hypersensitivity can be relieved by inactivating the intra-dental nerve and inhibiting neural transmission, using suitable medications .
It has been found that potassium-to-sodium intake ratios are strongly related to cardiovascular disease risk than either nutrient alone. The data describing this relationship warrants further research for various target tissue endpoints .
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Ingestion of large quantities may cause gastroenteritis. Chronic exposure can cause anemia, nephritis, and methemoglobinemia. When heated, reaction with calcium hydroxide + polychlorinated phenols forms extremely toxic chlorinated benzodtoxins. A powerful oxidizer. Gunpowder is a mixture of potassium nitrate + sulfur + charcoal. Explosive reaction with aluminum + barium nitrate + potassium perchlorate + water (in storage), boron + laminac + trichloroethylene. Forms explosive mixtures with lactose, powdered metals (e.g., titanium, antimony, germanium), metal sulfides (e.g., antimony trisulfide, barium sulfide, calcium sulfide, germanium monosulfide, titanium disulfide, arsenic disulfide, molybdenum disulfide), nonmetals (e.g., boron, carbon, white phosphorus, arsenic), organic materials, phosphides (e.g., copper(l1) phosphide, copper monophosphide), reducing agents (e.g., sodium phosphinate, sodium thiosulfate), sodium acetate. Can react violently under the appropriate conditions with 1,3- bis(trichlorometh~d)benzene, boron phosphde, F2, calcium shcide, charcoal, chromium nitride, Na hypophosphte, ma2O2 + dextrose), red phosphorus, (S + As2S3), thorium dicarbide, trichloroethylene, zinc, zirconium. When heated to decomposition it emits very toxic fumes of NOx and K2O. See also NITRATES.
Potential Exposure
Used to make explosives, gunpowder, fireworks, rocket fuel; matches, fertilizer, fluxes, glass manufacture; and as a diuretic
Metabolism
Nitrates are reduced to nitrites by the bacteria in saliva and the gastrointestinal system . The in vivo reduction of nitrates to nitrites depends on conditions that are subject to much variations such the volume and species of microflora present in the saliva/gastrointestinal tract, and stomach pH. Gastric pH is higher in infants younger than 6 months of age and during certain gastrointestinal tract infections, thereby favoring the reduction of nitrates .
Nitrate is metabolized to a small extent. The biotransformation of potassium nitrate consists of nitrate reduction, nitrite formation, nitrite reoxidation to nitrate, and formation of methemoglobin or NO, in a dynamic equilibrium , , .
Shipping
UN1486 Potassium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
It crystallises from hot H2O (0.5mL/g) on cooling (cf KNO2 below). Dry it for 12hours under vacuum at 70o. The solubility in H2O is 13.3% at 0o, 110% at 60o, and 246% at 100o. After two recrystallisations, technical grade salt had <0.001 ppm of metals. The fused salt is a powerful oxidising agent.
Incompatibilities
A powerful oxidizer. Dangerously reactive and friction-and shock-sensitive when mixed with organic materials and many materials. Violent reactions with reducing agents; chemically active metals; charcoal, trichloroethylene.
Properties of Potassium nitrate
| Melting point: | 334 °C (lit.) |
| Boiling point: | 100 °C750 mm Hg |
| Density | 1.00 g/mL at 20 °C |
| Flash point: | 400°C |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 2.109 |
| color | white |
| Odor | odorless, cooling pungent salty taste |
| PH | 5.0-7.5 (50g/l, H2O, 20℃) |
| Water Solubility | 320 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,7648 |
| Dielectric constant | 5(0.0℃) |
| Stability: | Stable. Strong oxidizer - contact with combustible material may cause fire. Incompatible with combustible materials, strong reducing agents. |
| CAS DataBase Reference | 7757-79-1(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium nitrate (7757-79-1) |
Safety information for Potassium nitrate
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P501:Dispose of contents/container to..… |
Computed Descriptors for Potassium nitrate
Potassium nitrate manufacturer
Mangalore Chemicals & Fertilizers Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

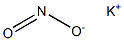
You may like
-
 Potassium CAS 7757-79-1View Details
Potassium CAS 7757-79-1View Details
7757-79-1 -
 Potassium CAS 7757-79-1View Details
Potassium CAS 7757-79-1View Details
7757-79-1 -
 Potassium nitrate, For ACS analysis CAS 7757-79-1View Details
Potassium nitrate, For ACS analysis CAS 7757-79-1View Details
7757-79-1 -
 Potassium nitrate, For ACS analysis CAS 7757-79-1View Details
Potassium nitrate, For ACS analysis CAS 7757-79-1View Details
7757-79-1 -
 Potassium nitrate CAS 7757-79-1View Details
Potassium nitrate CAS 7757-79-1View Details
7757-79-1 -
 Potassium nitrate CAS 7757-79-1View Details
Potassium nitrate CAS 7757-79-1View Details
7757-79-1 -
 Potassium nitrate CAS 7757-79-1View Details
Potassium nitrate CAS 7757-79-1View Details
7757-79-1 -
 Potassium Standard for ICP CASView Details
Potassium Standard for ICP CASView Details
