Cadmium chloride
Synonym(s):Cadmium dichloride;Dichlorocadmium
- CAS NO.:10108-64-2
- Empirical Formula: CdCl2
- Molecular Weight: 183.32
- MDL number: MFCD00010916
- EINECS: 233-296-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
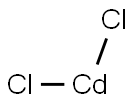
What is Cadmium chloride ?
Description
Cadmium chloride is a colourless and odourless crystal. It is used for the preparation of cadmium sulphide, used as ‘cadmium yellow’, a brilliant-yellow pigment, which is stable to heat and sulphide fumes. Cadmium chloride has a high solubility in water and is a non-combustible solid, but the dust can be a moderate fire hazard when exposed to heat or flame or when reacted with oxidising agents. It is incompatible with bromine trifluoride, potassium oxidisers, zinc, selenium, tellurium, and hydrogen azide.
Chemical properties
Milky White free flowing crystalline powder
Chemical properties
Cadmium chloride is a colorless, odorless, crystalline solid, or powder.
Physical properties
Colorless powder or crystal; hexagonal crystal system; hygroscopic; density 4.047 g/cm3; melts at 560°C; vaporizes at 960°C; highly soluble in water (140 g/100g at 20°C), also soluble in acetone; slightly soluble in alcohol; insoluble in ether.
The Uses of Cadmium chloride
Cadmium Chloride is used in the preparation of the stable inorganic pigment cadmium sulfide (Cadmium Yellow). Cadmium Chloride treatment of thin film CDTe solar cells is used to produce high-efficienc y solar cells.. Cadmium Chloride is also used in the preparation of organocadmium compounds.
The Uses of Cadmium chloride
Used in a preparation of highly luminescent CdTe nanocrystals.1
The Uses of Cadmium chloride
Preparation of cadmium standard; analysis of sulfides; testing of pyridine bases.
The Uses of Cadmium chloride
Made by the action of hydrochloric acid on cadmium and crystallization. The small white crystals are soluble in alcohol and water. Cadmium chloride was used to make collodionchloride printing-out emulsions, also known as leptographic or aristotype papers.
Production Methods
Cadmium chloride is manufactured in reaction of cadmium metal, carbonate, sulfide, oxide, or hydroxide with hydrochloric acid, followed by evaporation; it forms hydrated salt. Commercial cadmium chloride is amixture of hydrates that approximates to dihydrates. The commercial grade available in the United States typically contains about 51% of cadmium. Liquid caddy contains 20.1% cadmium chloride.
Preparation
Cadmium chloride may be prepared by heating the metal with chlorine or hydrogen chloride gas. In the solution, it is formed by treating the metal or its salts, such as oxide, hydroxide, carbonate, or sulfide with hydrochloric acid:
Cd + 2HCl → CdCl2 + H2
CdO + 2HCl → CdCl2 + H2O
CdCO3 + 2HCl → CdCl2+ H2O + CO2
The solution is evaporated and crystallized to yield a hydrated salt. The hydrated salt yields anhydrous cadmium chloride upon heating under hydrogen chloride or when refluxed with thionyl chloride.
Cadmium chloride also may be prepared by adding dry cadmium acetate to acetyl chloride in glacial acetic acid.
Definition
ChEBI: Cadmium dichloride is a cadmium coordination entity in which cadmium(2+) and Cl(-) ions are present in the ratio 2:1. Although considered to be ionic, it has considerable covalent character to its bonding.
General Description
Cadmium chloride is a white crystalline solid. Cadmium chloride is soluble in water. Cadmium chloride is noncombustible. The primary hazard of Cadmium chloride is that Cadmium chloride poses a threat to the environment. Immediate steps should be taken to limit its spread to the environment. Cadmium chloride is used in photography, in fabric printing, in chemical analysis, and in many other uses.
Air & Water Reactions
Water soluble.
Reactivity Profile
Bromine trifluoride rapidly attacks the following salts: barium chloride, Cadmium chloride , calcium chloride, cesium chloride, lithium chloride, silver chloride, rubidium chloride, potassium bromide, potassium chloride, potassium iodide, rhodium tetrabromide, sodium bromide, sodium chloride, and sodium iodide [Mellor 2 Supp. 1:164, 165 1956].
Health Hazard
Ingestion causes gastroenteric distress, pain, and prostration. Sensory disturbances, liver injury, and convulsions have been observed in severe intoxications.
Fire Hazard
Literature sources indicate that Cadmium chloride is nonflammable.
Safety Profile
Confirmed human carcinogen with experimental carcinogenic and tumorigenic data. Poison by ingestion, inhalation, skin contact, intraperitoneal, subcutaneous, intravenous, and possibly other routes. Human systemic effects by ingestion: blood pressure, acute pulmonary edema, hypermotility, diarrhea. Experimental teratogenic and reproductive effects. Human mutation data reported. Reacts violently with BrF3 and K. When heated to decomposition it emits very toxic fumes of Cd and Cl-. See also CADMIUM COMPOUNDS and CHLORIDES.
Potential Exposure
Cadmium chloride is used in dyeing and printing of fabrics; in electronic component manufacture; in photography; used as a pesticide and in nonpasture turf fungicides.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from cadmium.
storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Store in tightly closed containers.Avoid contact with strong acids and oxidizers or moisture.
Shipping
UN2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it from water (1mL/g) by addition of EtOH and cooling. [Pray Inorg Synth V 153 1957, Wagenknecht & Juza in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1093 1965.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, sulfur, selenium, potassium.
Waste Disposal
It is preferred to convert the salt to the nitrate, precipitate it with H2S, filter, wash and dry the precipitate and return it to the supplier.
Properties of Cadmium chloride
| Melting point: | 568 °C(lit.) |
| Boiling point: | 960 °C |
| Density | 1.01 g/mL at 20 °C |
| vapor density | 6.3 (vs air) |
| vapor pressure | 10 mm Hg ( 656 °C) |
| Flash point: | 960°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | beads |
| color | White |
| Specific Gravity | 4.047 |
| Water Solubility | 1400 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1617 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| Stability: | Stable. Reacts violently with bromine trifluoride and potassium. Incompatible with acids, oxidizing agents, sulfur, selenium and tellurium. Hygroscopic. |
| CAS DataBase Reference | 10108-64-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Cadmium dichloride(10108-64-2) |
| EPA Substance Registry System | Cadmium dichloride (10108-64-2) |
Safety information for Cadmium chloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H330:Acute toxicity,inhalation H340:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. |
Computed Descriptors for Cadmium chloride
Cadmium chloride manufacturer
Related products of tetrahydrofuran
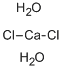
You may like
-
 Cadmium chloride 98%View Details
Cadmium chloride 98%View Details -
 Cadmium chloride CAS 10108-64-2View Details
Cadmium chloride CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride CAS 10108-64-2View Details
Cadmium chloride CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride, Ultra dry CAS 10108-64-2View Details
Cadmium chloride, Ultra dry CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride, Ultra dry CAS 10108-64-2View Details
Cadmium chloride, Ultra dry CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride, Anhydrous CAS 10108-64-2View Details
Cadmium chloride, Anhydrous CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride, Anhydrous CAS 10108-64-2View Details
Cadmium chloride, Anhydrous CAS 10108-64-2View Details
10108-64-2 -
 Cadmium chloride CASView Details
Cadmium chloride CASView Details