Sodium nitrate
Synonym(s):Sodium nitrate;Chile niter;Chile salpeter;Chile saltpeter, Soda niter;Nitric acid sodium salt
- CAS NO.:7631-99-4
- Empirical Formula: NNaO3
- Molecular Weight: 84.99
- MDL number: MFCD00011119
- EINECS: 231-554-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
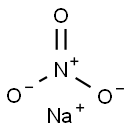
What is Sodium nitrate?
Description
Sodium nitrate, also known as Chile saltpeter and soda niter, has a molecular formula of NaNO3. Sodium nitrate is a colorless, odorless, transparent crystal. It oxidizes when exposed to air and is soluble in water. This material explodes at 1000°F (537°C), much lower than temperatures encountered in many fires. Sodium nitrate is toxic by ingestion and has caused cancer in test animals. When used in the curing of fish and meat products, it is restricted to 100 ppm. Sodium nitrate is incompatible with ammonium nitrate and other ammonium salts. The four-digit UN identification number is 1498. Sodium nitrate is used as an antidote for cyanide poisoning and in the curing of fish and meat.
Description
Sodium nitrate, also called saltpeter, is mined or synthetically produced and used as fertilizer, a food preservative in hot dogs and sausages, an ingredient in glass and ceramics. It was once of strategic importance because of its use in gunpowder.
Chemical properties
Sodium nitrate, NaNO3, also known as soda niter and Chile saltpeter, is a fire-hazardous, transparent, colorless and odorless crystalline solid. It is soluble in glycerol and water,decomposes when heated,and melts at 308°C (585 °F). Sodium nitrate is used in making nitric and sulfuric acids, in the manufacture of glass and pottery enamel, as a fertilizer, as a food preservative, in explosives, and as a welding flux.
Chemical properties
Sodium nitrate, white solid, soluble, source in nature is Chile, in the fixation of atmospheric nitrogen HNO3 is frequently transformed by sodium carbonate into sodium nitrate, and the solution evaporated. Used (1) as an important nitrogenous fertilizer, (2) as a source of nitrate and HNO3, (3) in pyrotechnics, (4) in fluxes.
Physical properties
Colorless crystalline solid; saline taste; trigonal, and rhombohedrals structure; density 2.257g/cm3; refractive index 1.587 (trigonal) and 1.336 (rhombohedral); melts at 308°C; decomposes at 380°C; specific conductance 95 μmhos/cm at 300°C; viscosity 2.85 centipoise at 317°C; very soluble in water 92.1 g/100 mL at 25°C and 180 g/100 mL at 100°C; very soluble in liquid ammonia; soluble in alcohol.
Occurrence
There are several natural deposits of sodium nitrate in various parts of the world, including Chile, Mexico, Egypt, and the United States. The most important application of sodium nitrate is its use as a fertilizer in agriculture. It is an effective fertilizer for cotton, tobacco, and vegetable crops. Its agricultural applications, however, have dwindled considerably in recent years because of the growth of ammonium nitrate and other fertilizers.
Another major use of sodium nitrate is in manufacturing explosives. It is a component of many types of dynamites and water-based slurry type blasting explosives. Sodium nitrate also is used in making charcoal briquettes. Sodium nitrate is used as an oxidizing and fluxing agent in manufacturing vitreous glass, fiberglass, porcelain, and enamels. Other uses are in the heat-treatment baths for alloys and metals, as a food preservative, in curing meats, and in preparing various salts.
The Uses of Sodium nitrate
In manufacturing of HNO{3}, as a catalyst to manufacture H{2}SO{4}, in manufacturing of glass, enamel for pottery, sodium nitriteSodium nitrate is used in the production of fertilizers, nitric acid, pyrotechnics, smoke-bombs, glass and pottery enamels. In combination with boron trifluoride it forms an efficient reagent for nitration of aromatic compounds. Adsorption on alumina provides an environmentally benign aromatic nitrating agent. Further it finds use as food preservative and as a solid rocket propellant. It is also used as an electrolyte in a salt bridge, and as thermal storage medium in power generation systems.
The Uses of Sodium nitrate
manufacture of nitric acid and as catalyst in the manufacture of sulfuric acid. manufacture of sodium nitrite, glass, enamels for pottery; in matches; for improving burning properties of tobacco; pickling meats; as color fixative in meats. Clinical reagent (parasites). The technical grade is used as fertilizer.
The Uses of Sodium nitrate
Sodium Nitrate is the salt of nitric acid that functions as an antimi- crobial agent and preservative. it is a naturally occurring substance in spinach, beets, broccoli, and other vegetables. it consists of color- less, odorless crystals or crystalline granules. it is moderately deli- quescent in moist air and is readily soluble in water. it is used in meat curing to develop and stabilize the pink color. see nitrate.
What are the applications of Application
Sodium nitrate is a water soluble compound that forms hexagonal crystals
Definition
ChEBI: The inorganic nitrate salt of sodium.
Production Methods
Sodium nitrate is recovered from natural deposits. One such process, known as the Guggenheim nitrate process, is briefly outlined below: The ore is crushed. Sodium nitrate is leached from the ore by extraction with a brine solution at 40°C. The brine for leaching is made up of an aqueous solution of magnesium sulfate, MgSO3, and calcium sulfate, CaSO3. The caliche variety of Chilean ore contains mostly sodium nitrate and sodium chloride as the main saline components, along with limestone, clays, sand, lime, and inert volcanic rocks. Sodium nitrate usually occurs in this ore as a double salt with sodium sulfate NaNO3?Na2SO3?H3O. This double salt, which is sparingly soluble in water, is broken down by magnesium in leaching brine solution, thus releasing more sodium nitrate into the extract. Sodium nitrate finally is recovered from the leachate brine by fractional crystallization.
Brines of other compositions have been used to extract sodium nitrate from its ores. Many such processes, including the Shanks process practiced in the past to produce sodium nitrate, are now obsolete.
General Description
A white crystalline solid. Noncombustible but accelerates the burning of combustible materials. If large quantities are involved in fire or the combustible material is finely divided an explosion may result. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in solid propellants, explosives, fertilizers, and for many other uses.
Air & Water Reactions
Soluble in water.
Reactivity Profile
A mixture of Sodium nitrate and sodium hypophosphite constitute a powerful explosive [Mellor 8, Supp. 1:154 1964]. Sodium nitrate and aluminum powder mixtures have been reported to be explosive,[Fire, 1935, 28, 30]. The nitrate appears to be incompatible with barium thiocyanate, antimony, arsenic trioxide/iron(II) sulfate, boron phosphide, calcium-sodium alloy, magnesium, metal amidosulfates, metal cyanides, powdered charcoal, peroxyformic acid, phenol/trifluoroacetic acid, sodium, sodium nitrite/sodium sulfide, sodium phosphinate, sodium thiosulfate, tris( cyclopentadienyl)cerium, and even wood [Bretherick 5th ed., 1995].
Hazard
Fire risk near organic materials, ignites on friction and explodes when shocked or heated to 1000F (537C). Toxic by ingestion; content in cured meats, fish, and other food products restricted.
Health Hazard
INGESTION: Dizziness, abdominal cramps, vomiting, bloody diarrhea, weakness, convulsions, and collapse. Small repeated doses may cause headache and mental impairment.
Flammability and Explosibility
Non flammable
Agricultural Uses
Sodium nitrate is the oldest known nitrogenous fertilizer.
It is a white, shiny crystal available in nature as Chilean
saltpeter or Chilean nitrate.
Sodium nitrate is manufactured by two methods. In
the first, known as the Guggenheim method, the
fertilizer is extracted from a mined product, called
caliche, mined mostly in Chile; hence the name (Chilean
saltpeter or Chilean nitrate). The caliche is dissolved in
warm water and then cooled to 0°C to produce sodium
nitrate crystals, which are circulated through heat
exchangers. The circulation keeps the crystals
suspended, to finally form pellets. Caliche mined in
Chile, contains sodium nitrate (8 to 20%), potassium and
magnesium nitrates and salts like borates, sulphates and
chlorides. Approximately, one ton of sodium nitrate of
99% purity is obtained from 10 tons of caliche. Sodium
nitrate is shipped in airtight containers. The pellets are
also coated to impart free-flowing characteristics.
Sodium nitrate is also manufactured from nitric acid
and soda ash, using salt and oyster shells. Nitric acid is
reacted with soda ash forming sodium nitrate solution.
Most water is removed by evaporation and the rest is heated to a high temperature and sprayed through
nozzles. Sodium nitrate solidifies as pellets while coming
through the nozzles.
Sodium nitrate fertilizer is water-soluble. It contains
16% nitrogen and about 26% sodium. Plants absorb most
of the nitrogen in a nitrate form and sodium nitrate is a
commonly preferred fertilizer, although the nitrogen
content of sodium nitrate is lesser than that in many other
inorganic nitrogen fertilizers. Sodium nitrate has a
neutralizing effect on soil acidity because of its inherent
basic residual effect. Its neutralizing value is 0.82 kg of
calcium carbonate equivalent to 0.45 kg of sodium
nitrate.
The field crops which benefit most from sodium
nitrate application are sugar beet and cotton. If applied
excessively, sodium nitrate can damage the soil structure
by reducing the flocculation. But normal applications of
100 to 200 kg of fertilizer/hectare/year do not affect the
soil structure.
Safety Profile
Human poison by ingestion. Poison by intravenous route.Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. A powerful oxidizer. It will iqte with heat or friction. Explodes when heated to over 1000°F, or when mixed with cyanides, sodium hypophosphte, boron phosphide. Forms explosive mixtures with aluminum powder, antimony powder, barium thiocyanate, metal amidosulfates, sodium, sodium phosphinate, sodium thiosulfate, sulfur + charcoal (gunpowder). Potentially violent reaction or ignition when mixed with bitumen, organic matter, calcium-shcon alloy, jute + magnesium chloride, magnesium, metal cyanides, nonmetals, peroxyformic acid, phenol + trifluoroacetic acid. Incompatible with acetic anhydride, barium thocyanate, wood. A dangerous disaster hazard. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and Na2O. See also NITRATES.
Purification Methods
Crystallise NaNO3 from hot water (0.6mL/g) by cooling to 0o, or from a concentrated aqueous solution by adding MeOH. Dry it under a vacuum at 140o. After two recrystallisations, technical grade sodium nitrate had K, Mg, B, Fe Al, and Li at 100, 29, 0.6, 0.4, 0.2 and 0.2 ppm respectively. (See KNO3.)
Properties of Sodium nitrate
| Melting point: | 306 °C (dec.) (lit.) |
| Boiling point: | 380 °C |
| Density | 1.1 g/mL at 25 °C |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | White powder |
| Specific Gravity | 2.261 |
| color | White or colorless |
| Odor | Odorless |
| PH | 5.5-8.0 (50g/l, H2O, 20℃) |
| Water Solubility | 900 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8647 |
| Dielectric constant | 5.2(0.0℃) |
| Stability: | Stable. Strong oxidizer - may ignite flammable material. Incompatible with cyanides, combustible material, strong reducing agents, aluminium. |
| CAS DataBase Reference | 7631-99-4(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium nitrate (7631-99-4) |
Safety information for Sodium nitrate
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sodium nitrate
Sodium nitrate manufacturer
Dhairya International
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Sodium nitrate CAS 7631-99-4View Details
Sodium nitrate CAS 7631-99-4View Details
7631-99-4 -
 Sodium nitrate CAS 7631-99-4View Details
Sodium nitrate CAS 7631-99-4View Details
7631-99-4 -
 Sodium nitrate CAS 7631-99-4View Details
Sodium nitrate CAS 7631-99-4View Details
7631-99-4 -
 Sodium nitrate, For ACS analysis CAS 7631-99-4View Details
Sodium nitrate, For ACS analysis CAS 7631-99-4View Details
7631-99-4 -
 Sodium nitrate, For ACS analysis CAS 7631-99-4View Details
Sodium nitrate, For ACS analysis CAS 7631-99-4View Details
7631-99-4 -
 Sodium Nitrate CASView Details
Sodium Nitrate CASView Details -
 Sodium Standard for AAS CASView Details
Sodium Standard for AAS CASView Details -
 Sodium Standard for ICP CASView Details
Sodium Standard for ICP CASView Details
