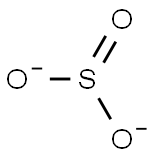
Nitrite
- Empirical Formula: NO2-
- Molecular Weight: 46.0055
- Update Date: 2025-01-27 09:38:02
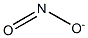
What is Nitrite?
The Uses of Nitrite
Nitrite is the salt of nitrous acid. It is used in meat curing to develop and stabilize the pink color associated with a cured meat and to affect flavor and function as an antioxidant. S convert to nitric oxide, which reacts with the myoglobin pigments (purple-red) to form nitrosomyoglobin (dark red). Nitrosomyoglobin plus heating to 130–140°f results in the formation of the stable pigment nitrosohemochrome, resulting in the cured meat color. It has bacteriostatic properties as an inhibitor of clostridia organisms, especially clostridium botulinum, and, therefore, nonsterile canned hams can be produced. Sources are sodium and potassium nitrite, with the sodium form being more commonly used.
Definition
A salt or ester of nitrous acid.
Definition
nitrite: A salt or ester of nitrousacid. The salts contain the dioxonitrate(III) ion, NO2-, which has a bondangle of 115°.
Safety information for Nitrite
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Nitrite CASView Details
Nitrite CASView Details -
 Nitrite CASView Details
Nitrite CASView Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
