beta-Pinene
Synonym(s):2(10)-Pinene;Nopinene;Pseudopinene;Terebenthene
- CAS NO.:127-91-3
- Empirical Formula: C10H16
- Molecular Weight: 136.23
- MDL number: MFCD00063635
- EINECS: 204-872-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
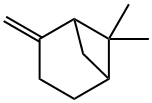
What is beta-Pinene?
Description
Beta-pinene is a component of turpentine. Concentrations vary depending on the source and seem to be high er in European (Portuguese) than in Asian (Indonesian) turpentines.
Description
β-Pinene, a bicyclic monoterpene, is the second-most abundant constituent of the resins produced by pine trees and other conifers. The most abundant is its isomer α-pinene1, the Molecule of the Week for May 14, 2012. Both pinenes exist in nature as their (+)- and (–)-enantiomers. The images show the (+)-enantiomer of β-pinene.
The pinenes are the chief ingredients of turpentine2, a solvent that was in widespread use as a diluent and cleaner when oil-based paints were in their heyday. It is also used in varnishes and as a raw material for synthesizing useful organic compounds.
Turpentine was mentioned in the chemical literature as early as 1875 in US Patent 162,394, in which Archibald K. Lee of Galveston, TX, used it in an improved process to dissolve “asphaltum”, a soluble form of asphalt. In 1893, Walter E. Rohner of New York City was awarded US 498,961 for its use in a “wood-polishing compound”.
β-Pinene itself was first identified in 1896 by noted German chemist Adolf von Baeyer in an extensive paper on the origins of terpenes. Baeyer, who also developed syntheses of indigo, phenolphthalein, and fluorescein, was awarded the 1905 Nobel Prize in Chemistry.
Much more information about β-pinene can be found in ScienceDirect’s information page on the molecule.
1. CAS Reg. No. 80-56-8.
2. CAS Reg. No. 9005-90-7.
Chemical properties
liquid
Chemical properties
β-Pinene occurs in many essential oils. Optically active and racemic β-pinenes are present in turpentine oils, although in smaller quantities than αα-pinene. (+)-β-Pinene, (1R,5R)-6,6-dimethyl-2-methylenebicyclo(3.1.1)heptane,; (?)-β-Pinene, (1S,5S)-6,6-dimethyl-2-methylenebicyclo(3.1.1)heptane,. β-Pinene is similar to α-pinene in its reactions. Pyrolytic cleavage to myrcene, the startingmaterial for acyclic terpenes, is used on an industrial scale.Addition of formaldehyde results in the formation of nopol; nopyl acetate is used as a fragrance material. β-Pinene is produced in large quantities by distillation of turpentine oils. It is used as a fragrance material in household perfumery.However,most β-pinene is used in the production of myrcene.
Chemical properties
β-Pinene has a characteristic turpentine odor with a dry, woody or resinous aroma.
Occurrence
Usually occurring together with α-pinene but in smaller amounts; the d- and l-forms are reported found in the essential oils of various Artemisae and several Cupressaceae, in coriander and cumin; the l-form is a constituent of several citrus oils. β-Pinene is reported found in over 190 natural products including apple, apricot, many citrus juices and peel oils, bilberry, cranberry, lingonberry, blackberry, currants, guava, raspberry, strawberry, basil, carrot, celery, cooked potato, bell pepper, tomato, anise seed oil, cinnamon, cassia leaf, clove, cumin, ginger, Mentha oils, nutmeg, mace, pepper, parsley, thyme, Swiss and cheddar cheese, cream, fatty fish, fried chicken, beef fat, hop oil, rum, bourbon whiskey, tea, roasted filberts, pecans, oats, soybean, plum, mushroom, sweet and wild marjoram, starfruit, mango, tamarind, cardamom, coriander, gin, rice, litchi, calamus, dill, lovage, caraway seed, buckwheat, laurel, fennel, kiwifruit, myrtle leaf and berry, rosemary, buchu oil, Bourbon vanilla, Spanish and clary sage, nectarine, crayfish, clam, cape gooseberry, anise hyssop, angelica root oil, Roman and German chamomile oil, eucalyptus oil, bullock’s heart and mastic gum leaf and fruit oil.
Definition
ChEBI: An isomer of pinene with an exocyclic double bond. It is a component of essential oils from many plants.
Preparation
Isolated from American turpentine; also by conversion from α-pinene; as an intermediate extremely important for the manufacture of citral, citronellol, hydroxycitronellal, geraniol, citronellal, linalool, ionones, methylionones and menthol.
Aroma threshold values
Detection: 140 ppb. Aroma characteristics at 10%: cooling, woody, piney and turpentine-like with a fresh minty, eucalyptus and camphoraceous note with a spicy peppery and nutmeg nuance.
Taste threshold values
Taste characteristics at 15 to 100 ppm: fresh, piney and woody, terpy and resinous with a slight minty, spicy and camphoraceous nuance.
Contact allergens
Beta-pinene is a component of turpentine. Concentrations vary with the source and seem higher in European (Portuguese) than in Asian (Indonesian) turpentine.
Safety Profile
Mddly toxic by ingestion. A skin irritant. Flammable liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of beta-Pinene
| Melting point: | -61°C |
| Boiling point: | 167°C |
| Density | 0.859 |
| refractive index | 1.4782 |
| FEMA | 2903 | BETA-PINENE |
| Flash point: | 43°C |
| solubility | insoluble |
| appearance | colorless liquid |
| Odor | at 10.00 % in dipropylene glycol. dry woody resinous pine hay green eucalyptus camphoreous |
| Water Solubility | 12mg/L(25 ºC) |
| Merck | 7446 |
| JECFA Number | 1330 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 127-91-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Beta-pinene(127-91-3) |
| EPA Substance Registry System | .beta.-Pinene (127-91-3) |
Safety information for beta-Pinene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for beta-Pinene
beta-Pinene manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 127-91-3 Beta Pinene 99%View Details
127-91-3 Beta Pinene 99%View Details
127-91-3 -
 127-91-3 98%View Details
127-91-3 98%View Details
127-91-3 -
 Beta-Pinene 98%View Details
Beta-Pinene 98%View Details
127-91-3 -
 Beta-Pinene 127-91-3 98%View Details
Beta-Pinene 127-91-3 98%View Details
127-91-3 -
 Beta-Pinene 127-91-3 98%View Details
Beta-Pinene 127-91-3 98%View Details
127-91-3 -
 127-91-3 Beta-Pinene 98%View Details
127-91-3 Beta-Pinene 98%View Details
127-91-3 -
 127-91-3 98%View Details
127-91-3 98%View Details
127-91-3 -
 beta-Pinene 95% (GC) CAS 127-91-3View Details
beta-Pinene 95% (GC) CAS 127-91-3View Details
127-91-3
