Azelaic acid
Synonym(s):1,7-Heptanedicarboxylic acid;Anchoic acid;Azelaic acid;Nonanedioic acid
- CAS NO.:123-99-9
- Empirical Formula: C9H16O4
- Molecular Weight: 188.22
- MDL number: MFCD00004432
- EINECS: 204-669-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
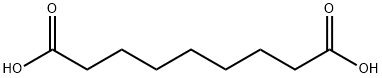
What is Azelaic acid?
Absorption
Approximately 4% of the topically applied azelaic acid is systemically absorbed.
Toxicity
Oral LD50 in rat: >5 g/kg
Description
Azelaic acid is a topical antiacne agent which exerts its therapeutic action through a myriad of antimicrobial, antiproliferative and cytostatic effects. In vitro, azelaic acid hasbeen shown to inhibit DNA polymerases in several tumor cell lines.
Description
Azelaic acid, formally nonanedioic acid, is a white crystalline solid with a melting point of 106.5 °C. It occurs naturally in grains such as wheat, rye, and barley and is produced industrially by ozonolyzing oleic acid. It is used topically to treat acne and rosacea. Some plants release azelaic acid as a "distress flare" to signal cells to activate their defenses against attacking pathogens.
Chemical properties
white to cream solid
Chemical properties
Azelaic acid is an organic compound with the formula (CH2)7(CO2H)2. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a component of a number of hair and skin conditioners.
Chemical properties
Nonanedioic acid is the best known dicarboxylic acid. Its name stems from the action of nitric acid (azote, nitrogen, or azotic, nitric) oxidation of oleic or elaidic acid. It was detected among products of rancid fats. Its origin explains for its presence in poorly preserved samples of linseed oil and in specimens of ointment removed from Egyptian tombs 5000 years old. Azelaic acid was prepared by oxidation of oleic acid with potassium permanganate, but now by oxidative cleavage of oleic acid with chromic acid or by ozonolysis. Azelaic acid is used, as simple esters or branched-chain esters) in the manufacture of plasticizers (for vinyl chloride resins, rubber), lubricants and greases. Azelaic acid is now used in cosmetics (treatment of acne). It displays bacteriostatic and bactericidal properties against a variety of aerobic and anaerobic micro-organisms present on acne-bearing skin. Azelaic acid was identified as a molecule that accumulated at elevated levels in some parts of plants and was shown to be able to enhance the resistance of plants to infections.
Originator
Schering AG (W. Germany)
The Uses of Azelaic acid
Azelaic acid is used in lacquers, alkyd resins, plasticizers, adhesives, polyamides, urethane elastomers, and organic syntheses. Azelaic acid is also used in treating of acne.
The Uses of Azelaic acid
antiacne, antiproliferative agent
The Uses of Azelaic acid
antifungal, binds to membrane sterols
Background
Azelaic acid is a saturated dicarboxylic acid found naturally in wheat, rye, and barley. It is also produced by Malassezia furfur, also known as Pityrosporum ovale, which is a species of fungus that is normally found on human skin. Azelaic acid is effective against a number of skin conditions, such as mild to moderate acne, when applied topically in a cream formulation of 20%. It works in part by stopping the growth of skin bacteria that cause acne, and by keeping skin pores clear. Azelaic acid's antimicrobial action may be attributable to inhibition of microbial cellular protein synthesis.
Indications
For the topical treatment of mild-to-moderate inflammatory acne vulgaris.
What are the applications of Application
Azelaic acid is an antiinflammatory compound
Production Methods
Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid.
What are the applications of Application
Polymers and related materials
Esters of this dicarboxylic acid find applications in lubrication and plasticizers. With hexamethylenediamine azelaic acid forms Nylon - 6,9, which finds specialized uses as a plastic.
Medical
Azelaic acid is used to treat mild to moderate acne, both comedonal acne and inflammatory acne . It belongs to a class of medication called dicarboxylic acids. It works by killing acne bacteria that infect skin pores. It also decreases the production of keratin, which is a natural substance that promotes the growth of acne bacteria Azelaic acid is also used as a topical gel treatment for rosacea, due to its ability to reduce inflammation . It clears the bumps and swelling caused by Rosacea. Azelaic acid has been used for treatment of skin pigmentation including melasma and post inflammatory hyper pigmentation , particularly in those with darker skin types. It has been recommended as an alternative to hydroquinone (HQ). As a tyrosinase inhibitor, azelaic acid reduces synthesis of melanin.
Brand names
AzClear Action ( 20 % lotion, Ego Pharmaceuticals), Azelex (20% cream, Allergan), White Action cream (20 % cream ,2 % glycolic acid), SynCare), Finacea (15 % gel, Intendis/Berlex Laboratories, subsidiaries of Bayer AG), Finevin (20 % cream, Intendis / Berlex Laboratories), Skinoren (20 % cream or 15% gel, Intendis), Melazepam, Strata Dermatologics, 2oz, Mixed Dicarboxylic Acids 20 % Azelaic Acid Cream.,and Azaclear (azelaic acid and niacinamide, Epikinetics LLC). .
Indications
Azelaic acid (Azelex) is a naturally occurring dicarboxylic acid produced by the yeast Malassezia furfur. Azelaic acid inhibits tyrosinase, a rate-limiting enzyme in the synthesis of the pigment melanin. This may explain why diminution of melanin pigmentation occurs in the skin of some patients with pityriasis versicolor, a disease caused by M. furfur. Azelaic acid is bacteriostatic against a number of species thought to participate in the pathogenesis of acne, including Propionibacterium acnes. The drug may also reduce microcomedo formation by promoting normalization of epidermal keratinocytes.
What are the applications of Application
Azelaic acid, also known as azalea acid, is a white to slightly yellow powder. Azelaic acid is a medium-long chain dibasic acid[1]. In recent years, with the rapid development of the organic synthetic chemical industry, the demand for medium and long chain dibasic acids is increasing. The medium and long chain dibasic acids and their derivatives have a wide range of industrial applications and a broad product market.
Preparation
Azelaic acid is made by the ozonolysis of oleic acid:
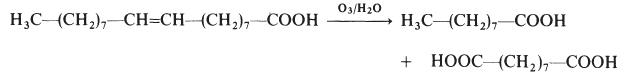
Definition
ChEBI: Nonanedioic acid is an alpha,omega-dicarboxylic acid that is heptane substituted at positions 1 and 7 by carboxy groups. It has a role as an antibacterial agent, an antineoplastic agent, a dermatologic drug and a plant metabolite. It is a dicarboxylic fatty acid and an alpha,omega-dicarboxylic acid. It is a conjugate acid of an azelaate(2-) and an azelaate.
Manufacturing Process
Two step oxidation of tall oil fatty acid using peroxyformic acid and nitric
acid/sodium metavanadate were used to produce azelaic acid.
Step 1 (derivatization of the double bond):
A hydroxy acyloxy derivative of tall oil fatty acid (TOFA) was prepared by
mixing 200 g of TOFA (63% oleic acid, 31% linoleic acid) with 500 mL of formic acid. The resulting mixture was vigorously stirred by magnetic action.
Hydrogen peroxide solution, 180 mL of 35% by weight, was added in aliquots
to the mixture throughout the course of the reaction. A third of the total
amount of peroxide solution was added at once to initiate the reaction. The
peroxyformic acid in this case was prepared in situ.
The start of the reaction was signalled by heat evolution and a dramatic color
change, from pale yellow to deep rust red. The exothermicity of the reaction
required external cooling to control the temperature. The reaction was
maintained at 40°C to minimize oxygen loss through the decomposition of the
peroxide. As required, the temperature of the reaction was maintained with an
external heating source. A total reaction time of 5 to 6 hours was necessary
for complete reaction. The end of the reaction was indicated by a color
change, the reaction mixture changed from rust red back to yellow. One last
aliquot of peroxide solution was added at the end of the reaction period to
provide a peroxide atmosphere during the reaction work-up. TOFA as a
substrate produced a mixture of mono- and dihydroxy formoxystearic acid
from the oleic and linoleic acid components, respectively. The final product
was obtained in essentially 100% yield by removing the unreacted formic acid
and hydrogen peroxide as well as water. It was obtained as a viscous, syrupy
yellow oil that upon gas chromatographic analysis of the methyl esters of the
reaction mixture gave no evidence of unreacted substrate.
Step 2 (oxidation of derivative obtained from step 1):
A 2 L three neck flask fitted with an air condenser attached to a gas scrubbing
apparatus was filled with 500 mL of concentrated nitric acid (70% by weight).
The acid was stirred by magnetic action and 1 g of sodium metavanadate was
added to it. The resulting mixture was heated slowly to 40°-50°C. At this
point a small amount of product as obtained from Step 1 was added to the
acid-catalyst mixture. Heating was continued until a sharp temperature
increase accompanied by evolution of NOx gases was observed. The reaction
temperature was self-sustained with the addition of aliquots of the hydroxy
formoxy ester mixture obtained from Step 1. (External cooling may be
required throughout the substrate addition period to keep the temperature
within 65°-70°C). At the end of the addition period the reaction temperature
was maintained for an additional 1.5 to 2 hours, for a total reaction time of 3
hours.
The final products were obtained by quenching the reaction by adding excess
water and extracting the organic layer with purified diethyl ether. The ether
extract was dried over anhydrous sodium sulfate overnight before its removal
with a roto-vap apparatus. Addition of petroleum ether (boiling range 35°-
60°C) to the product mixture caused precipitation of the diacid component.
Vacuum filtration was used to remove the solid diacids from the liquid
monoacid mixture. The latter was obtained by removing the excess petroleum
ether from the resulting filtrate. Quantitative analysis by gas chromatography
of the methyl esters showed that the products to be 96% yield of diacid (66%
azelaic, 30% suberic).
brand name
Azelex (Allergan); Finacea (Intendis);Skinoren.
Therapeutic Function
Antiacne, Depigmentor
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 4846, 1955 DOI: 10.1021/ja01623a048
Organic Syntheses, Coll. Vol. 2, p. 53, 1943
General Description
Azelaic acid is used as a therapeutic agent in dermatology.
Biochem/physiol Actions
Azelaic acid is a potent inhibitor of 5α-reductase activity. It is a reversible competitive inhibitor of thioredoxin reductase in human melanoma cells.
Biotechnological Applications
In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response.
Mechanism of action
Naturally occurring dicarboxylic acid that is bacteriostatic to Propionibacterium acnes. It also decreases conversion of testosterone to 5{pi}ga-dihydrotestosterone (DHT) and alters keratinization of the microcomedone. It may also be beneficial in the treatment of melasma. The mechanism of action is not fully understood. Deoxyribonucleic acid (DNA) synthesis is reduced, and mitochondrial cellular energy products are inhibited in melanocytes.
Pharmacokinetics
Azelaic acid is a saturated dicarboxylic acid found naturally in wheat, rye, and barley. It is a natural substance that is produced by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. It is effective against a number of skin conditions, such as mild to moderate acne, when applied topically in a cream formulation of 20%. It works in part by stopping the growth of skin bacteria that cause acne, and by keeping skin pores clear. Azelaic acid's antimicrobial action may be attributable to inhibition of microbial cellular protein synthesis.
Clinical Use
Azelaic acid is used for the treatment of mild to moderate acne, particularly in cases characterized by marked inflammation-associated hyperpigmentation.
Benefits
Azelaic acid has antioxidant, antibacterial and anti-inflammatory properties, which have an inhibitory effect on bacteria on acne-prone skin, reducing inflammation, which in turn protects the skin. In addition, it scavenges free radicals, reduces hyperpigmentation, prevents clogging of pores and helps prevent vasodilation, thus minimising redness and improving skin tone, among other benefits.
Safety Profile
Low toxicity by ingestion. A skinand eye irritant. Closely related to glutaric acid and adipicacid. Combustible when exposed to heat or flame; canreact with oxidizing materials.
Side Effects
Azelaic acid may cause burning or stinging, itching, dryness, acne, peeling and reddening of the skin when first used. Other serious side effects include crusting, pain, measles, severe redness, swelling, Scaliness and asthma.
Metabolism
Mainly excreted unchanged in the urine but undergoes some b-oxidation to shorter chain dicarboxylic acids.
Purification Methods
Recrystallise it from H2O(charcoal) or thiophene-free *benzene. The acid can be dried by azeotropic distillation with toluene, the residual toluene solution is then cooled and filtered, and the precipitate is dried in a vacuum oven. It has been purified by zone refining or by sublimation onto a cold finger at 10-3torr. It distils above 360o with partial formation of the anhydride. The dimethyl ester has m –3.9o and b 140o/8mm. [Hill & McEwen Org Synth Coll Vol II 53 1943, Beilstein 2 IV 2055.]
Properties of Azelaic acid
| Melting point: | 98 °C |
| Boiling point: | 286 °C100 mm Hg(lit.) |
| Density | 1,029 g/cm3 |
| vapor density | 6.5 (vs air) |
| vapor pressure | <1 mm Hg ( 20 °C) |
| refractive index | 1.4303 |
| Flash point: | 215 °C |
| storage temp. | Store below +30°C. |
| solubility | 2.4g/l |
| form | Slightly Crystalline Powder or Flakes |
| pka | 4.53, 5.33(at 25℃) |
| color | White to slightly yellow |
| PH | 3.5 (1g/l, H2O) |
| Water Solubility | 2.4 g/L (20 ºC) |
| Merck | 14,905 |
| BRN | 1101094 |
| Stability: | Stable. Combustible. Incompatible with bases, strong oxidizing agents. Readily biodegrades in soil and water with >70% DOC reduction after 28 days. |
| CAS DataBase Reference | 123-99-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Nonanedioic acid(123-99-9) |
| EPA Substance Registry System | Azelaic acid (123-99-9) |
Safety information for Azelaic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Azelaic acid
| InChIKey | BDJRBEYXGGNYIS-UHFFFAOYSA-N |
Azelaic acid manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Azelaic Acid 98%View Details
Azelaic Acid 98%View Details -
 Azelaic acid 99%View Details
Azelaic acid 99%View Details -
 Azelaic acid CAS 123-99-9View Details
Azelaic acid CAS 123-99-9View Details
123-99-9 -
 Azelaic acid CAS 123-99-9View Details
Azelaic acid CAS 123-99-9View Details
123-99-9 -
 Azelaic acid CAS 123-99-9View Details
Azelaic acid CAS 123-99-9View Details
123-99-9 -
 Azelaic acid CAS 123-99-9View Details
Azelaic acid CAS 123-99-9View Details
123-99-9 -
 Azelaic acid CAS 123-99-9View Details
Azelaic acid CAS 123-99-9View Details
123-99-9 -
 Azelaic Acid ApiView Details
Azelaic Acid ApiView Details
123-99-9
