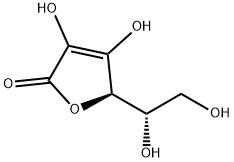
Ascoric Acid
- CAS NO.:36431-82-0
- Empirical Formula: C32H42N2O7
- Molecular Weight: 566.7
- MDL number: MFCD01941285
- EINECS: 202-303-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:16:29

What is Ascoric Acid?
Description
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form ("vitamer") of vitamin C. It was originally called L-hexuronic acid, but when it was found to have vitamin C activity in animals ("vitamin C" being defined as a vitamin activity, not then a specific substance), the suggestion was made to rename L-hexuronic acid. The new name for L-hexuronic acid is derived from a- (meaning "no") and scorbutus (scurvy), the disease caused by a deficiency of vitamin C. Because it is derived from glucose, many animals are able to produce it, but humans require it as part of their nutrition. Other vertebrates lacking the ability to produce ascorbic acid include other primates, guinea pigs, teleost fishes, bats,and birds, all of which require it as a dietary micronutrient (that is, a vitamin).
Chemical properties
Ascorbic acid is present in nutritionally useful amounts in many edible plants, especially in rapidly growing leafy vegetables, fruits, tomatoes and potatoes. Foods of animal origin as usually consumed are generally poor sources of the vitamin. Of the stereoisomers (L-ascorbic acid, D-ascorbic acid, L-isoascorbic acid and D-isoascorbic acid or erythorbic acid), only L-ascorbic acid has significant vitamin C activity. The vitamin C activity of ascorbyl palmitate is approximately equal to that of L-ascorbic acid. Ascorbic acid has a pleasant, sharp, acidic taste without any odor. It is extensively used as antioxidant, meat-curing aid, nutrient and dietary supplement. For a detailed description, see Burdock (1997).
Occurrence
Reported found in rose hip, black currants, the juice of citrus fruits and the ripe fruit of Capsicum annuum L.
History
From the middle of the 18th century, it was noted that lemon juice could prevent sailors from getting scurvy. At first, it was supposed that the acid properties were responsible for this benefit; however, it soon became clear that other dietary acids, such as vinegar, had no such benefits. In 1907, two Norwegian physicians reported an essential disease-preventing compound in foods that was distinct from the one that prevented beriberi. These physicians were investigating dietary deficiency diseases using the new animal model of guinea pigs, which are susceptible to scurvy. The newly discovered food-factor was eventually called vitamin C.
From 1928 to 1932, the Hungarian research team led by Albert Szent-Gy?rgyi, as well as that of the American researcher Charles Glen King, identified the antiscorbutic factor as a particular single chemical substance. At the Mayo clinic, Szent-Gy?rgyi had isolated the chemical hexuronic acid from animal adrenal glands. He suspected it to be the antiscorbutic factor, but could not prove it without a biological assay. This assay was finally conducted at the University of Pittsburgh in the laboratory of King, which had been working on the problem for years, using guinea pigs. In late 1931, King's lab obtained adrenal hexuronic acid indirectly from Szent-Gy?rgyi and using their animal model, proved that it is vitamin C, by early 1932.
The Uses of Ascoric Acid
Vitamin (antiscorbutic); acidifier (urinary).
The Uses of Ascoric Acid
ascorbic acid (vitamin C) is ascorbic acid and its derivatives, such as ascorbyllinoleate, are said to have skin-lightening and antioxidant properties. Its stability is a main concern when incorporating it into cosmetic formulations.
The Uses of Ascoric Acid
It is termed vitamin C, a water-soluble vitamin that prevents scurvy, helps maintain the body’s resistance to infection, and is essential for healthy bones and teeth. It is the most easily destroyed vitamin and processing is recommended in stainless steel or glass. Storage at below ?18°C is recommended. In its dry form it is nonreactive, but in solution it readily reacts with atmospheric oxygen and other oxidizing agents. One part is equivalent to one part erythorbic acid. It is used as a vitamin supplement in beverages, potato flakes, and breakfast foods; and as a dough conditioning agent to strengthen and condition bread roll doughs. It is also used as an antioxidant to increase shelf life in canned and frozen processed foods. It is used in conjunction with BHA, BHT, and propyl gallate to regenerate them following the chemical changes they undergo when they prevent fat rancidity in bologna and other meats. Other forms of are isoascorbic (erythorbic) acid, sodium ascorbate, and sodium isoascorbate.
The Uses of Ascoric Acid
Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
In fluorescence microscopy and related fluorescence - based techniques, ascorbic acid can be used as an antioxidant to increase fluorescent signal and chemically retard dye photobleaching.
It is also commonly used to remove dissolved metal stains, such as iron, from fiberglass swimming pool surfaces.
In plastic manufacturing, ascorbic acid can be used to assemble molecular chains more quickly and with less waste than traditional synthesis methods.
Preparation
Ascorbic acid is prepared industrially from glucose in a method based on the historical Reichstein process. In the first of a five-step process, glucose is catalytically hydrogenated to sorbitol, which is then oxidized by the microorganism Acetobacter suboxydans to sorbose. Only one of the six hydroxy groups is oxidized by this enzymatic reaction. From this point, two routes are available. Treatment of the product with acetone in the presence of an acid catalyst converts four of the remaining hydroxyl groups to acetals. The unprotected hydroxyl group is oxidized to the carboxylic acid by reaction with the catalytic oxidant TEMPO (regenerated by sodium hypochlorite — bleaching solution). (Historically, industrial preparation via the Reichstein process used potassium permanganate.) Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization. This step yields ascorbic acid. Each of the five steps has a yield larger than 90 %.
Reactions
Ascorbic acid resembles the sugar from which it is derived, being a ring with many oxygen-containing functional groups. The molecule exists in equilibrium with two ketone tautomers, which are less stable than the enol form . In solutions, these forms of ascorbic acid rapidly interconvert.
Nucleophilic attack of ascorbic enol on proton to give 1,3-diketone.
brand name
Ascorbicap (ICN); Cebione (Abbott); Cecon (Abbott); Cenolate (Abbott); Cetane (Forest); Cetane-Caps TC (Forest); Cevalin (Lilly); Cevex (Marion Merrell Dow).
Biosynthesis
Ascorbic acid is found in plants and animals where it is produced from glucose. Animals must either produce it or digest it, otherwise a lack of vitamin C may cause scurvy which may eventually lead to death. Reptiles and older orders of birds make ascorbic acid in their kidneys. Recent orders of birds and most mammals make ascorbic acid in their liver where the enzyme L-gulono lactone oxidase is required to convert glucose to ascorbic acid. Humans, some other primates, and guinea pigs are not able to make L-gulono lactone oxidase because of a genetic mutation and are therefore unable to make ascorbic acid. Synthesis and signalling properties are still under investigation.
Biotechnological Production
Traditionally, ascorbic acid is produced via the Reichstein process, which is a chemical synthesis route with several process steps. In the last 20 years, the development of biotechnological processes for ascorbic acid has been in focus. More information about ascorbic acid and its utilization in beverage, food, and animal nutrition as well as its biotechnological production are offered in Industrial Production of L-Ascorbic Acid (Vitamin C) and D-Isoascorbic Acid.
Mechanism of action
As a mild reducing agent, ascorbic acid degrades upon exposure to air, converting the oxygen to water. The redox reaction is accelerated by the presence of metal ions and light. It can be oxidized by one electron to a radical state or doubly oxidized to the stable form called dehydroascorbic acid.
Ascorbate usually acts as an antioxidant. It typically reacts with oxidants of the reactive oxygen species, such as the hydroxyl radical formed from hydrogen peroxide. Such radicals are damaging to animals and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. Sometimes these radicals initiate chain reactions. Ascorbate can terminate these chain radical reactions by electron transfer. Ascorbic acid is special because it can transfer a single electron, owing to the stability of its own radical ion called "semidehydroascorbate", dehydroascorbate. The net reaction is :
RO? + C6H7O6- → ROH + C6H6O6?-
The oxidized forms of ascorbate are relatively unreactive, and do not cause cellular damage.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
Acidity
Ascorbic acid, a reductone, behaves as a vinylogous carboxylic acid wherein the electrons in the double bond, hydroxyl group lone pair, and the carbonyl double bond form a conjugated system. Because the two major resonance structures stabilize the deprotonated conjugate base of ascorbic acid, the hydroxyl group in ascorbic acid is much more acidic than typical hydroxyl groups. In other words, ascorbic acid can be considered an enol in which the deprotonated form is a stabilized enolate.
Synthesis
Ascorbic acid is prepared synthetically or extracted from various vegetable sources in which it occurs naturally. Synthesis involves the hydrogenation of D-glucose to D-sorbitol, followed by oxidation of the diacetone derivative of L-sorbose and the resulting diacetone-2-keto-l-gulonic acid converted to L-ascorbic acid by heating with hydrochloric acid.
Food chemistry
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water-soluble and thus cannot protect fats from oxidation: For this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants. Eighty percent of the world's supply of ascorbic acid is produced in China. this is very bad for you espetially when heaed in food .
Properties of Ascoric Acid
| pka | 4.1(at 24℃) |
Safety information for Ascoric Acid
Computed Descriptors for Ascoric Acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8