Stearic acid
Synonym(s):Stearic acid;Octadecanoic acid;STA;C18:0;Cetylacetic acid
- CAS NO.:57-11-4
- Empirical Formula: C18H36O2
- Molecular Weight: 284.48
- MDL number: MFCD00002752
- EINECS: 266-928-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
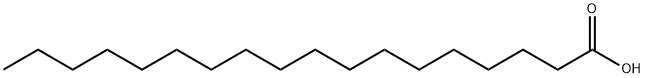
What is Stearic acid?
Toxicity
Acute oral toxicity (LD50): 4640 mg/kg [Rat]. Acute dermal toxicity (LD50): >5000 mg/kg Rabbit.
Description
Stearic acid is a long-chain saturated fatty acid. It is a major component of cocoa butter and has also been found in beef fat and vegetable oils. Unlike many long-chain saturated fatty acids, dietary stearic acid does not induce hypercholesterolemia or raise LDL-cholesterol.
Chemical properties
Stearic acid is a hard, white or faintly yellow-colored, somewhat glossy, crystalline solid or a white or yellowish white powder. It has a slight odor (with an odor threshold of 20 ppm) and taste suggesting tallow.
Occurrence
Stearic acid is naturally present in the glycerides of animal fats and most vegetable oils. Reported found in fresh apple, banana, Vitis vinifera L., melon, tomato, ginger, blue cheeses, cheddar cheese, Swiss cheese, feta cheese, buttermilk, raw fatty fish, raw lean fish, raw shrimp, grapefruit juice, guava, papaya, cucumber, saffron, pork and lamb liver, pork fat, hop oil, beer, cognac, rum, whiskies, sherry, tea, peanut oil, soybean, roast coconut, coconut milk, avocado, passion fruit, rose apple, mushroom, starfruit, fenugreek, mango, cardamom, cooked rice, prickly pear, dill seed, buckwheat, malt, wort, cassava, loquat, shrimp, crab, cape gooseberry and Chinese quince.
The Uses of Stearic acid
Stearic Acid is an anti-inflammatory agent that induces ICAM-1 expression. It is the main ingredient used in making bar soaps and lubricants. It occurs naturally in butter acids, tallow, cascarilla bark, and in other animal fats and oils. Stearic acid may cause allergic reactions in people with sensitive skin and is considered somewhat comedogenic.
Background
Stearic acid (IUPAC systematic name: octadecanoic acid) is one of the useful types of saturated fatty acids that comes from many animal and vegetable fats and oils. It is a waxy solid.
Definition
ChEBI: A C18 straight-chain saturated fatty acid component of many animal and vegetable lipids. As well as in the diet, it is used in hardening soaps, softening plastics and in making cosmetics, candles and plastics.
Production Methods
Stearic acid is manufactured by hydrolysis of fat by continuous
exposure to a countercurrent stream of high-temperature water and
fat in a high-pressure chamber. The resultant mixture is purified by
vacuum steam distillation and the distillates are then separated
using selective solvents.
Stearic acid may also be manufactured by the hydrogenation of
cottonseed and other vegetable oils; by the hydrogenation and
subsequent saponification of olein followed by recrystallization
from alcohol; and from edible fats and oils by boiling with sodium
hydroxide, separating any glycerin, and decomposing the resulting
soap with sulfuric or hydrochloric acid. The stearic acid is then
subsequently separated from any oleic acid by cold expression.
Synthesis
Stearic Acid occurs in many animal and vegetable fats and oils, but it is more abundant in animal fat (up to 30 %) than vegetable fat (typically < 5 % ) . The important exceptions are cocoa butter and shea butter where the stearic acid content (as a triglyceride) is 28 – 45 %.
Stearic acid is prepared by treating these fats and oils with water at a high pressure and temperature (above 200 °C), leading to the hydrolysis of triglycerides. The resulting mixture is then distilled. Commercial stearic acid is often a mixture of stearic and palmitic acids, although purified stearic acid is available.
In terms of its biosynthesis, stearic acid is produced from carbohydrates via the fatty acid synthesis machinery via acetyl-CoA .
brand name
Hystrene 5016 (Witco).
Aroma threshold values
Detection: 20 ppm
Synthesis Reference(s)
Synthetic Communications, 15, p. 759, 1985 DOI: 10.1080/00397918508063869
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Stearic acid is incompatible with strong oxidizers and strong bases. Stearic acid is also incompatible with reducing agents.
Health Hazard
Compound is generally considered nontoxic. Inhalation of dust irritates nose and throat. Dust causes mild irritation of eyes.
Fire Hazard
Stearic acid is combustible. Stearic acid can heat spontaneously.
Pharmaceutical Applications
Stearic acid is widely used in oral and topical pharmaceutical
formulations. It is mainly used in oral formulations as a tablet and
capsule lubricant, although it may also be used as a
binder or in combination with shellac as a tablet coating. It has
also been suggested that stearic acid may be used in enteric tablet
coatings and as a sustained-release drug carrier.
In topical formulations, stearic acid is used as an emulsifying and
solubilizing agent. When partially neutralized with alkalis or
triethanolamine, stearic acid is used in the preparation of
creams. The partially neutralized stearic acid forms a creamy
base when mixed with 5–15 times its own weight of aqueous liquid,
the appearance and plasticity of the cream being determined by the
proportion of alkali used.
Stearic acid is used as the hardening agent in glycerin
suppositories.
Stearic acid is also widely used in cosmetics and food products.
Biochem/physiol Actions
β-Oxidation of stearic acid yields eight FADH2 (flavin adenine dinucleotide) and NADH2 (nicotinamide adenine dinucleotide) molecules and nine acetyl-CoA molecules.
Safety
Stearic acid is widely used in oral and topical pharmaceutical
formulations; it is also used in cosmetics and food products. Stearic
acid is generally regarded as a nontoxic and nonirritant material.
However, consumption of excessive amounts may be harmful.
LD50 (mouse, IV): 23 mg/kg
LD50 (rat, IV): 21.5 mg/kg
Stearic Acid application
Stearic Acid is a fatty acid that is a mixture of solid organic acids obtained principally from stearic acid and palmitic acid. it is practi- cally insoluble in water. it functions as a lubricant, binder, and defoamer. it is used as a softener in chewing gum base.
Metabolism
An isotope labeling study in humans concluded that the fraction of dietary stearic acid oxidatively desaturated to oleic acid was 2.4 times higher than the fraction of palmitic acid analogously converted to palmitoleic acid. Also, stearic acid was less likely to be incorporated into cholesterol esters. In epidemiologic and clinical studies stearic acid was associated with lowered LDL cholesterol in comparison with other saturated fatty acids. These findings may indicate that stearic acid is healthier than other saturated fatty acids.
Storage
Stearic acid is a stable material; an antioxidant may also be added to it. The bulk material should be stored in a wellclosed container in a cool, dry place.
Purification Methods
Crystallise stearic acid from acetone, acetonitrile, EtOH (5 times), aqueous MeOH, ethyl methyl ketone or pet ether (b 60-90o), or by fractional precipitation by dissolving in hot 95% EtOH and pouring into distilled water, with stirring. The precipitate, after washing with distilled water, is dried under vacuum over P2O5. It has also been purified by zone melting and partial freezing. [Tamai et al. J Phys Chem 91 541 1987, Beilstein 2 IV 1206.]
Incompatibilities
Stearic acid is incompatible with most metal hydroxides and may be
incompatible with bases, reducing agents, and oxidizing agents.
Ointment bases made with stearic acid may show evidence of
drying out or lumpiness due to such a reaction when compounded
with zinc or calcium salts.
A number of differential scanning calorimetry studies have
investigated the compatibility of stearic acid with drugs. Although
such laboratory studies have suggested incompatibilities, e.g. with
naproxen, they may not necessarily be applicable to formulated
products.
Stearic acid has been reported to cause pitting in the film coating
of tablets applied using an aqueous film-coating technique; the
pitting was found to be a function of the melting point of the stearic
acid.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe (fatty acids). Included in the FDA Inactive Ingredients Database (sublingual tablets; oral capsules, solutions, suspensions, and tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Stearic acid
| Melting point: | 67-72 °C (lit.) |
| Boiling point: | 361 °C (lit.) |
| Density | 0.845 g/cm3 |
| vapor pressure | 1 mm Hg ( 173.7 °C) |
| refractive index | 1.4299 |
| FEMA | 3035 | STEARIC ACID |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Practically insoluble in water, soluble in ethanol (96 per cent) and in light petroleum (bp: 50-70 °C). |
| pka | pKa 5.75±0.00(H2O
t = 35) (Uncertain) |
| form | powder |
| color | White |
| Specific Gravity | 0.84 (80℃) |
| Odor | odorless mild fatty |
| Water Solubility | 0.1-1 g/100 mL at 23 ºC |
| Merck | 14,8804 |
| JECFA Number | 116 |
| BRN | 608585 |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 |
| Dielectric constant | 2.3(22℃) |
| CAS DataBase Reference | 57-11-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Octadecanoic acid(57-11-4) |
| EPA Substance Registry System | Stearic acid (57-11-4) |
Safety information for Stearic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Stearic acid
| InChIKey | QIQXTHQIDYTFRH-UHFFFAOYSA-N |
Stearic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 STEARIC ACID 99%View Details
STEARIC ACID 99%View Details -
 Stearic Acid 98%View Details
Stearic Acid 98%View Details -
 STEARIC ACID 98%View Details
STEARIC ACID 98%View Details -
 Stearic Acid 99%View Details
Stearic Acid 99%View Details -
 Stearic Acid 99%View Details
Stearic Acid 99%View Details -
 STEARIC ACID 99%View Details
STEARIC ACID 99%View Details -
 Stearic Acid (SQ) CAS 57-11-4View Details
Stearic Acid (SQ) CAS 57-11-4View Details
57-11-4 -
 Stearic Acid CASView Details
Stearic Acid CASView Details
