AZD-9291
- CAS NO.:1421373-65-0
- Empirical Formula: C28H33N7O2
- Molecular Weight: 499.61
- MDL number: MFCD27988062
- EINECS: 629-848-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
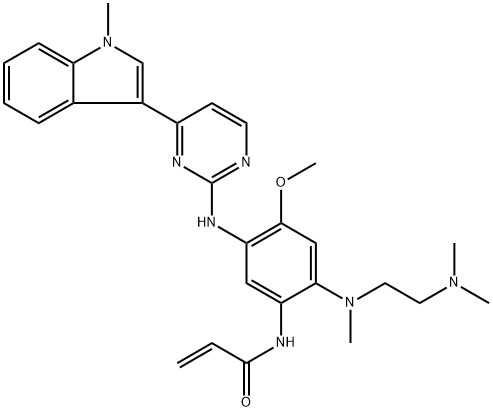
What is AZD-9291?
Absorption
The median time to Cmax was found to be 6 hours.
Toxicity
Across clinical trials, interstitial lung disease (ILD)/pneumonitis occurred in 3.7% of treated patients with 0.3% of these being fatal. There is also a change of QTc interval prolongation; electrocardiogram and electrolytes should be monitored in patients with a history or predisposition for QTc prolongation. Cardiomyopathy occurred in 3% of patients, therefore left ventricular ejection fraction (LVEF) should be measured at baseline and during treatment. Osimertinib can cause embryo-fetal toxicity, requiring female patients to take effective birth control during therapy and for 6 weeks after final dose.
Description
AZD-9291 (Mesylate), also known as Osimertinib, belongs to a third-generation EGFR (epidermal growth factor receptor) inhibitor. It can be used for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) in case when the cancer cells carry specific mutations (such as T790M) in the epidermal growth factor receptor. It takes effect through directly binding to the certain mutant forms of EGFR (T790M, L858R, and exon 19 deletions) that are frequently found in NSCLC, further causing poor prognosis for late-stage disease. Since it spares wild type EGFR during therapy, it has only limited toxicity.
The Uses of AZD-9291
AZD 9291 is an irreversible inhibitor of epidermal growth factor receptor (EGFR) sensitizing and T790M resistance mutations (IC50s = 15-17 nM) while sparing the wild-type form of the receptor (IC50 = 480 nM). It binds the related IGF1R and hERG receptors with significantly reduced potency (IC50s = 2.9 and 16.2 μM, respectively). AZD 9291 has been shown to inhibit tumor growth in a xenograft mouse model at oral doses of 5-10 mg/kg and has been tested clinically in patients with advanced EGFR mutant non-small-cell lung cancer.
Background
Osimertinib is an oral, third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug developed by AstraZeneca Pharmaceuticals. Its use is indicated for the treatment of metastatic non-small cell lung cancer (NSCLC) in cases where tumour EGFR expression is positive for the T790M mutation as detected by FDA-approved testing and which has progressed following therapy with a first-generation EGFR tyrosine kinase inhibitor. Approximately 10% of patients with NSCLC have a rapid and clinically effective response to EGFR-TKIs due to the presence of specific activating EGFR mutations within the tumour cells. More specifically, deletions around the LREA motif in exon 19 and exon 21 L858R point mutations are correlated with response to therapy.
Development of third-generation EGFR-TKIs, such as osimertinib, has been in response to altered tumour resistance patterns following treatment and toxic side effects that impact patient quality of life. Treatment with first-generation EGFR-TKIs (gefitinib and erlotinib) has been associated with the development of resistance through activating mutations in the EGFR gene. Second-generation EGFR-TKIs (afatinib and dacomitinib) were then developed to be more potent inhibitors, although their use is associated with increased toxicity through nonspecific targeting of wild-type EGFR. In contrast, third-generation inhibitors are specific for the gate-keeper T790M mutations which increases ATP binding activity to EGFR and result in poor prognosis for late-stage disease. Furthermore, osimertinib has been shown to spare wild-type EGFR during therapy, thereby reducing non-specific binding and limiting toxicity.
Indications
Osimertinib is indicated as adjuvant therapy after tumor resection in adult patients with non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R mutations (as detected by an FDA-approved test), and as the first-line treatment of adult patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations (as detected by an FDA-approved test). Osimertinib is also indicated for the treatment of adult patients with metastatic EGFR T790M mutation-positive NSCLC, as detected by an FDA-approved test, whose disease has progressed on or after EGFR TKI therapy.
Definition
ChEBI: A member of the class of aminopyrimidines that is 4-(1-methylindol-3-yl)pyrimidin-2-amine in which one of the amino hydrogens is replaced by a 2-methoxy-4-[2-(dimethylamino)ethyl](methyl)amino-5-acrylamidophenyl group. Used (as the mesylate salt) for treat ent of EGFR T790M mutation positive non-small cell lung cancer.
Indications
The collection of ibrutinib (Imbruvica(R), Pharmacyclics Inc.), afatinib, and osimertinib represents the small, yet expanding, group of covalent SMKIs. Ibrutinib is a non-receptor Bruton’s tyrosine kinase inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia. Afatinib, approved for NSCLC in 2013 and squamous NSCLC in 2016, is a second-generation irreversible EGFR inhibitor that targets wild-type EGFR, the mutant T790M EGFR, and HER2. Osimertinib (AZD9291), which was approved by FDA in November 2015, is a third-generation irreversible EGFR inhibitor that selectively targets the mutant T790M EGFR. Rociletinib, which shares a high degree of structural similarity with that of osimertinib, is a promising covalent EGFR inhibitor developed by Clovis Oncology aimed for the treatment of patients with EGFR T790M-mutated NSCLC, until the company terminated its development in May 2016 following a negative vote fromthe FDA’sOncologic Drugs Advisory Committee.
Pharmacokinetics
A pharmacokinetic/pharmacodynamic analysis suggested a concentration-dependent QTc interval prolongation of 14 msec (upper bound of two-sided 90% CI: 16 msec) at a dose of osimertinib 80 mg.
Mechanism of action
Osimertinib (AZD-9291) is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that binds to certain mutant forms of EGFR (T790M, L858R, and exon 19 deletion) that predominate in non-small cell lung cancer (NSCLC) tumours following treatment with first-line EGFR-TKIs. As a third-generation tyrosine kinase inhibitor, osimertinib is specific for the gate-keeper T790M mutation which increases ATP binding activity to EGFR and results in poor prognosis for late-stage disease. Furthermore, osimertinib has been shown to spare wild-type EGFR during therapy, thereby reducing non-specific binding and limiting toxicity. Compared to wild-type EGFR, osimertinib has 200 times higher affinity for EGFR molecules with the L858R/T790M mutation in vitro.
Metabolism
Osimertinib is metabolized to at least two pharmacologically active metabolites, AZ7550 and AZ5104, that circulate at approximately 10% of the concentration of the parent compound. Biochemical assays have shown that AZ7550 has similar potency and efficacy to osimertinib, while AZ5104 is more potent against mutant and wild-type EGFR. The main metabolic pathways are oxidation (predominantly by CYP3A) and dealkylation.
References
http://www.medkoo.com/products/6711
https://en.wikipedia.org/wiki/Osimertinib
https://www.drugbank.ca/drugs/DB09330
Properties of AZD-9291
| Melting point: | >188oC (dec.) |
| Density | 1.19±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | 12.68±0.70(Predicted) |
| form | Solid |
| color | Pale Yellow to Beige |
| CAS DataBase Reference | 1421373-65-0 |
Safety information for AZD-9291
Computed Descriptors for AZD-9291
AZD-9291 manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 1421373-65-0 Osimertinib Base 98%View Details
1421373-65-0 Osimertinib Base 98%View Details
1421373-65-0 -
 1421373-65-0 Mereletinib 98%View Details
1421373-65-0 Mereletinib 98%View Details
1421373-65-0 -
amino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide 98% CAS 1421373-65-0](https://img.chemicalbook.in//Content/image/CP5.jpg) N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide 98% CAS 1421373-65-0View Details
N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide 98% CAS 1421373-65-0View Details
1421373-65-0 -
 AZD-9291 95% (HPLC) CAS 1421373-65-0View Details
AZD-9291 95% (HPLC) CAS 1421373-65-0View Details
1421373-65-0 -
 1421373-65-0 95.00%View Details
1421373-65-0 95.00%View Details
1421373-65-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
