Afatinib (BIBW 2992)
- CAS NO.:439081-18-2
- Empirical Formula: C24H25ClFN5O3
- Molecular Weight: 485.94
- MDL number: MFCD12407405
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
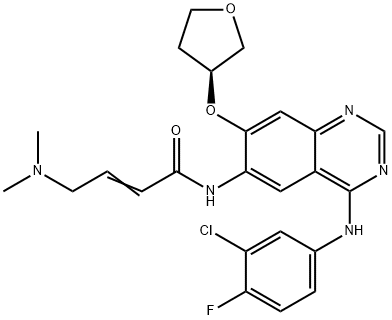
What is Afatinib (BIBW 2992)?
Description
In July 2013, the US FDA approved afatinib (also referred to as BIBW- 2992), for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) and with tumors that have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution. Afatinib functions as an irreversible inhibitor by covalently binding directly to the ATP-binding site in the kinase domains of both EGFR(Cys 773) andHER2 (Cys 805;HER-2 is the preferred dimerization partner of EGFR) resulting in downregulation of EGFR signaling. Afatinib is a potent inhibitor of wild-type and mutant forms (L858R) of EGFR (IC50s of 0.5 and 0.4 nM, respectively), and HER2 (IC50=14 nM), but about 100-fold more active against the gefitinib resistant L858R– T790M EGFR double mutant, with an IC50 of 10 nM. Consistent with its in vitro activity, afatinib induces tumor regression in xenograft and transgenic lung cancer models, with superior activity over erlotinib. A synthetic route to afatinib that employs the displacement of a phenylsulfonyl group to install the (S)-3-hydoxytetrahydrofuran ring and a modified Horner–Wadsworth–Emmons reaction with {[4-(3-chloro-4- phenylamino)-7-((S)-tetrahydrofuran-3-yloxy)-quinazolin-6-ylcarbamoyl]- methyl}-phosphonate and dimethylaminoacetaldehyde-hydrogen sulfite adduct to install the eneamide moiety, has been reported.
Originator
Boehringer-Ingelheim (United States)
The Uses of Afatinib (BIBW 2992)
BIBW2992 (Afatinib, Tomtovok, Tovok) irreversibly inhibits EGFR/HER2 including EGFRwt, EGFRL858R, EGFRL858R/T790M and HER2 with IC50 of 0.5 nM, 0.4 nM, 10 nM and 14 nM, respectively.
What are the applications of Application
Afatinib is an irreversible and potent EGFR/Neu inhibitor, inhibits EGFR phosphorylation.
Definition
ChEBI: Afatinib is a quinazoline compound having a 3-chloro-4-fluoroanilino group at the 4-position, a 4-dimethylamino-trans-but-2-enamido group at the 6-position, and an (S)-tetrahydrofuran-3-yloxy group at the 7-position. Used (as its dimaleate salt) for the first-line treatment of patients with metastatic non-small cell lung cancer. It has a role as a tyrosine kinase inhibitor and an antineoplastic agent. It is a member of quinazolines, a member of furans, an organofluorine compound, an enamide, an aromatic ether, a tertiary amino compound, a member of monochlorobenzenes and a secondary carboxamide.
brand name
Gilotrif
Properties of Afatinib (BIBW 2992)
| Boiling point: | 676.9±55.0 °C(Predicted) |
| Density | 1.380 |
| storage temp. | Store at -20°C |
| solubility | ≥24.3 mg/mL in DMSO; insoluble in H2O; ≥42.1 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 12.06±0.43(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 439081-18-2 |
Safety information for Afatinib (BIBW 2992)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Afatinib (BIBW 2992)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![4-QuinazolinaMine, N-(3-chloro-4-fluorophenyl)-6-nitro-7-[[(3S)-tetrahydro-3-furanyl]oxy]-](https://img.chemicalbook.in/CAS/GIF/314771-88-5.gif)

You may like
-
 Afatinib 99% (HPLC) CAS 439081-18-2View Details
Afatinib 99% (HPLC) CAS 439081-18-2View Details
439081-18-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
