Apremilast
- CAS NO.:608141-41-9
- Empirical Formula: C22H24N2O7S
- Molecular Weight: 460.5
- MDL number: MFCD18782607
- EINECS: 807-237-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 16:50:29
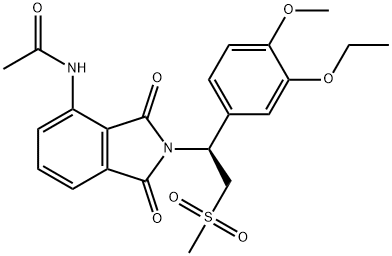
What is Apremilast?
Absorption
An oral dose of apremilast is well-absorbed and the absolute bioavailability is approximately 73%. Tmax is approximately 2.5 hours and Cmax has been reported to be approximately 584 ng/mL in one pharmacokinetic study. Food intake does not appear to affect apremilast absorption.
Toxicity
The oral LD50 in mice was greater than 2000 mg/kg in mice. In rats, oral LD50 was 2000 mg/kg males and 300 mg/kg in females.
Overdose information
In healthy subjects receiving a maximum dose of 100 mg (given as 50 mg twice daily) for about 5 days, no significant toxicity was observed. In cases of an overdose, supportive and symptomatic treatment should be administered. Contact the local poison control center for the most recent overdose management for apremilast.
Description
Apremilast is the first and only oral phosphodiesterase IV (PDE- 4) inhibitor and anti-tumor necrosis factor alpha (TNFa) agent launched in the USA by Celgene for the treatment of active psoriatic arthritis (PsA). Apremilast was also approved for the treatment of moderate to severe plaque psoriasis in the USA. Later, this drug was also approved in the European Union (EU) for both indications. Although multiple approaches to the synthesis of apremilast have been described, the most likely process scale approach involves the construction of the challenging stereogenic benzylic carbon center by catalytic asymmetric hydrogenation.
The Uses of Apremilast
Apremilast is an oral phosphodiesterase 4 inhibitor used in the treatment of adults with active psoriatic arthritis and adults with moderate to severe plaque psoriasis.
Indications
Apremilast is indicated for the treatment of adults with active psoriatic arthritis and adults with oral ulcers associated with Behcet's Disease. In addition, apremilast is indicated for the treatment of plaque psoriasis, of any severity, in adult patients who are candidates for phototherapy or systemic therapy.
Background
Apremilast, also known as Otezla, is a phosphodiesterase 4 (PDE4) inhibitor used to treat various types of symptoms resulting from certain inflammatory autoimmune diseases. It belongs to the same drug class as Roflumilast and Crisaborole. Initially approved in 2014, it is marketed by Celgene. In July 2019, apremilast was granted a new FDA approval for the treatment of oral ulcers associated with Behcet's disease, an autoimmune condition that causes recurrent skin, blood vessel, and central nervous system inflammation.
Definition
ChEBI: Apremilast is a member of the class of isoindoles that is isoindole-1,3-dione substituted at position 4 by an acetamido group and at position 1 by a 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl group. Used for treatment of psoriatic arthritis. It has a role as a phosphodiesterase IV inhibitor and a non-steroidal anti-inflammatory drug. It is an aromatic ether, a N-acetylarylamine, a sulfone and a member of phthalimides.
Pharmacokinetics
Apremilast reduces but does not completely inhibit various inflammatory cytokines such as IL-1α, IL-6, IL-8, IL-10 MCP-1, MIP-1β, MMP-3, and TNF-α, relieving the symptoms of psoriasis and Behcet's disease, which are caused by an increase in these inflammatory mediators. This drug has also been proven to be effective in relieving the pain associated with oral ulcers in Behcet's disease.
Apremilast may cause unwanted weight loss and worsen depression, leading to suicidal thoughts or actions. It is advisable to monitor for symptoms of depression and seek medical attention if they occur, especially in patients with pre-existing depression. The need for apremilast should be carefully assessed along with the risk of worsening depression and suicide. If weight loss occurs, the degree of weight loss should be evaluated, and consideration should be made for the possible discontinuation of apremilast.
Clinical Use
Treatment of active psoriatic arthritis (PsA) and moderate to severe chronic plaque psoriasis (PSOR)
Synthesis
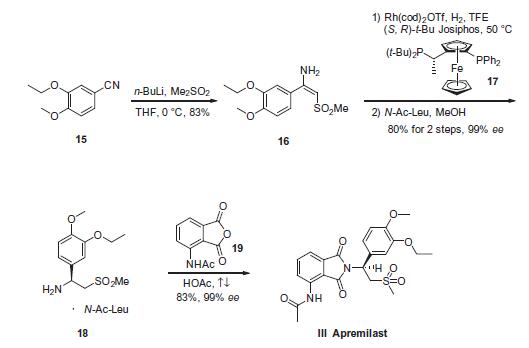
Dimethyl sulfone was first subjected to n-butyllithium in tetrahydrofuran (THF) prior to exposure to commercially available 3-ethoxy-4-methoxybenzonitrile (15) at low temperature to afford enamine 16 in 83% yield, presumably as a single isomer enamine possessing the E-configuration. After considerable studies conducted by researchers at Celgene regarding the reduction of this enamine and alternative substrates, enamine 16 was reduced under asymmetric hydrogenation conditions consisting of [Rh (COD)2]OTf and (S,R)-t-Bu Josiphos in trifluoroethanol (TFE) at 50 ?? under 90 psi of hydrogen pressure. Immediate exposure of the product to N-acetyl-L-leucine in methanol afforded the corresponding benzylamine salt 18 in 80% yield and more than 99% enantiomeric excess (ee). Finally, compound 18 was condensed with commercially available N-(phthalimid-3-yl)acetamide (19) in refluxing acetic acid to provide apremilast (III) in 83% yield and 99.4% ee.
Overdosage
Apremilast was studied in healthy subjects at a maximum total daily dose of 100 mg (given as 50 mg twice daily) for 4.5 days without evidence of dose limiting toxicities. In case of an overdose, it is recommended that the patient is monitored for any signs or symptoms of adverse effects and appropriate symptomatic treatment is instituted. In the event of overdose, symptomatic and supportive care is advised.
Enzyme inhibitor
This thalidomide-like psoriasis drug (FW = 460.50 g/mol; CAS 608141-41-9; Solubility: 90 mg/mL DMSO; <1 mg/mL H2O), also known as CC-10004, Otezla? and N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methyl-sulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]acetamide, is a potent, orally active phosphodiesterase inhibitor that targets phosphodiesterase PDE4 (IC50 = 74 nM), itself a proinflammatory mediator, and TNF-α, IC50 = 77 nM. Apremilast inhibits PBMC production of the chemokines CXCL9 and CXCL10, cytokines interferon-γ and tumor necrosis factor-α (TNF-α), and interleukins IL-2, IL-12 and IL- 23 from human rheumatoid synovial membrane cultures. Apremilast significantly reduces clinical scores in both murine models of arthritis over a ten-day treatment period and maintains healthy joint architecture in a dose-dependent manner. Unlike rolipram, however, apremilast demonstrated no adverse behavioral effects in na?ve mice. Otezla is specifically indicated by the Food & Drug Administration for the treatment of patients with moderate to severe plaque psoriasis who are typically candidates for phototherapy or systemic therapy. That said, the specific mechanism(s) for therapeutic action in psoriatic arthritis patients and psoriasis patients is unclear.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid.
Metabolism
Apremilast is heavily metabolized by various pathways, which include oxidation, hydrolysis, in addition to conjugation. About 23 metabolites are produced from its metabolism. The CYP3A4 primarily mediates the oxidative metabolism of this drug, with smaller contributions from CYP1A2 and CYP2A6 enzymes. The main metabolite of apremilast, M12, is an inactive glucuronide conjugate form of the O-demethylated drug. Some other major metabolites, M14 and M16, are significantly less active in the inhibition of PDE4 and inflammatory mediators than their parent drug, apremilast. After an oral dose, unchanged apremilast (45%) and the inactive metabolite, O-desmethyl apremilast glucuronide (39%) are found in the plasma. Minor metabolites M7 and M17 are active, but are only present in about 2% or less of apremilast concentrations, and likely not significant contributors to the actions of apremilast.
Metabolism
Apremilast is extensively metabolised by both CYP and non-CYP mediated pathways including oxidation, hydrolysis, and conjugation, suggesting inhibition of a single clearance pathway. After oral administration of radiolabelled apremilast, about 3% and 7% of the radioactive dose is recovered as apremilast in urine and faeces, respectively.
Mode of action
Apremilast, an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4), works intracellularly to modulate a network of pro-inflammatory and anti-inflammatory mediators. PDE4 is a cyclic adenosine monophosphate (cAMP)-specific PDE and the dominant PDE in inflammatory cells. PDE4 inhibition elevates intracellular cAMP levels, which in turn down-regulates the inflammatory response by modulating the expression of TNF-α, IL-23, IL-17 and other inflammatory cytokines. Cyclic AMP also modulates levels of anti-inflammatory cytokines such as IL-10. These pro- and anti-inflammatory mediators have been implicated in psoriatic arthritis and psoriasis.
Properties of Apremilast
| Melting point: | 152-156°C |
| Boiling point: | 741.3±60.0 °C(Predicted) |
| Density | 1.381 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 14.01±0.20(Predicted) |
| color | White to Pale Yellow |
| CAS DataBase Reference | 608141-41-9 |
Safety information for Apremilast
Computed Descriptors for Apremilast
| InChIKey | IMOZEMNVLZVGJZ-QGZVFWFLSA-N |
| SMILES | C(NC1=CC=CC2=C1C(=O)N([C@@H](C1=CC=C(OC)C(OCC)=C1)CS(C)(=O)=O)C2=O)(=O)C |
Apremilast manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![N-(2,2,2-Trifluoroethyl)-9-(4-[4-[4'-(trifluoromethyl)[1,1'-biphenyl]-2-carboxamido]piperidin-1-yl]butyl)-9H-fluorene-9-carboxamide](https://img.chemicalbook.in/CAS/20180808/GIF/182431-12-5.gif)

You may like
-
 Apremilast 98%View Details
Apremilast 98%View Details -
 Apremilast 97%View Details
Apremilast 97%View Details -
 608141-41-9 Apremilast 98%View Details
608141-41-9 Apremilast 98%View Details
608141-41-9 -
 Apremilast 608141-41-9 98%View Details
Apremilast 608141-41-9 98%View Details
608141-41-9 -
 Apremilast 98%View Details
Apremilast 98%View Details -
 Apremilast 98%View Details
Apremilast 98%View Details -
 Apremilast 97% CAS 608141-41-9View Details
Apremilast 97% CAS 608141-41-9View Details
608141-41-9 -
 Apremilast 99% (HPLC) CAS 608141-41-9View Details
Apremilast 99% (HPLC) CAS 608141-41-9View Details
608141-41-9