Pazopanib
Synonym(s):5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)(methyl)amino]pyrimidin-2-yl]amino]-2-methylbenzenesulfonamide;5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide;GW 786034;GW786034
- CAS NO.:444731-52-6
- Empirical Formula: C21H23N7O2S
- Molecular Weight: 437.52
- MDL number: MFCD11616589
- EINECS: 251-228-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
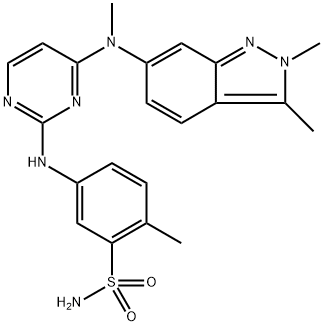
What is Pazopanib?
Absorption
Absorption of pazopanib in cancer patients is slow and incomplete. In patients with solid tumour, over a dose range of 50-2000 mg, absorption is nonlinear. Significant accumulation of pazopanib can also be observed in patients receiving 800 mg once daily for 22 days. Crushing tablets may increase exposure (increase in Cmax and AUC, while Tmax decreases by 2 hours). Bioavailability, oral tablet 800 mg, cancer patient = 21%; Bioavailability may be low due to incomplete absorption from the gastrointestinal tract. The major circulating component of the drug in the systemic is pazopanib, and not its metabolites. Mean maximum plasma concentration= 58.1 μg/mL; Mean AUC= 1037 μg · h/mL;
Description
Pazopanib is a multi-kinase inhibitor that inhibits the VEGF receptors VEGFR1, VEGFR2, and VEGFR3 (IC50s = 10, 30, and 47 nM, respectively, in a cell-free enzyme assay). It also inhibits PDGFRα, PDGFRβ, and c-Kit (IC50s = 71, 84, and 74 nM, respectively, in a cell-free enzyme assay) as well as additional receptor tyrosine kinases. Pazopanib inhibits upregulation of the surface adhesion proteins ICAM-1 and VCAM-1 induced by VEGF in multiple myeloma cells cocultured with human umbilical vein endothelial cells (HUVECs) and decreases multiple myeloma cell adhesion to HUVECs. It also inhibits proliferation of multiple myeloma cells cocultured with HUVECs. Pazopanib (30 and 100 mg/kg) reduces tumor growth, induces apoptosis, decreases angiogenesis, and increases survival in a multiple myeloma mouse xenograft model. Formulations containing pazopanib have been used in the treatment of cancer.
Chemical properties
Off-White Solid
Characteristics
Class: receptor tyrosine kinase
Treatment: RCC, STS
Oral bioavailability = 14–39%
Elimination half-life = 31 h
Protein binding > 99.9%
The Uses of Pazopanib
Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively.
The Uses of Pazopanib
An oral angiogenesis inhibitor targeting VEGFR and PDGFR.
The Uses of Pazopanib
Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR-α/β, and c-Kit.
The Uses of Pazopanib
Pazopanib is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively.
Background
Pazopanib is a small molecule inhibitor of multiple protein tyrosine kinases with potential antineoplastic activity. It is developed by GlaxoSmithKline and was FDA approved on October 19, 2009.
Indications
Treatment of advanced renal cell cancer and advanced soft tissue sarcoma (in patients previously treated with chemotherapy)
What are the applications of Application
Pazopanib is a potent and selective inhibitor of Flk-1, PDGFR and c-kit
Definition
ChEBI: A pyrimidine that is 5-(pyrimidin-2-yl}amino-2-methylbenzenesulfonamide substituted at position 4 by a (2,3-dimethylindazol-6-yl)(methyl)amino group. Used as its hydrochloride salt for treatment of kidney cancer.
Pharmacokinetics
Pazopanib is a synthetic indazolylpyrimidine and reaches steady state concentrations of >15 μg/ml. This concentration is high enough to observe maximal inhibition of VEGFR2 phosphorylation and some anti-tumour activity (concentration required to inhibit receptors is 0.01 - 0.084 μmol/L). A reduction in tumour blood flow, increased tumour apoptosis, inhibition of tumour growth, reduction in tumour interstitial fluid pressure, and hypoxia in cancer cells can be observed in patients receiving treatment.
Clinical Use
Tyrosine kinase inhibitor:
Treatment of metastatic renal cell carcinoma and soft
tissue sarcoma
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with clarithromycin, rifampicin
and telithromycin.
Antifungals: avoid with itraconazole, ketoconazole
and voriconazole.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid with atazanavir, boceprevir,
indinavir, ritonavir and saquinavir.
Grapefruit juice: avoid concomitant administration.
Avoid concomitant use with other inhibitors or
inducers of CYP3A4. Dose alterations may be
required.
Metabolism
Metabolized by CYP3A4 and to a lesser extent by CYP1A2 and CYP2C8. Metabolites are less active than pazopanib (10 to 20-fold less active). Three of its metabolites can be observed in the systemic and account for <10% of plasma radioactivity.
Metabolism
Metabolism primarily by CYP3A4, with minor
contributions from CYP1A2 and CYP2C8. The four
principle pazopanib metabolites account for only 6% of
the exposure in plasma. One of these metabolites inhibits
the proliferation of VEGF-stimulated human umbilical
vein endothelial cells with a similar potency to that
of pazopanib, the others are 10- to 20-fold less active.
Therefore, activity of pazopanib is mainly dependent on
parent pazopanib exposure.
Elimination is mostly via the faeces.
Properties of Pazopanib
| Melting point: | 285-289°C (dec.) |
| Boiling point: | 728.8±70.0 °C(Predicted) |
| Density | 1.40 |
| Flash point: | 359℃ |
| storage temp. | Refrigerator |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | 10.19±0.60(Predicted) |
| form | Yellow powder. |
| color | White to Beige |
Safety information for Pazopanib
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Pazopanib
Pazopanib manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 444731-52-6 Pazopanib 98%View Details
444731-52-6 Pazopanib 98%View Details
444731-52-6 -
 444731-52-6 99%View Details
444731-52-6 99%View Details
444731-52-6 -
 Pazopanib 98%View Details
Pazopanib 98%View Details
444731-52-6 -
 Pazopanib 444731-52-6 98%View Details
Pazopanib 444731-52-6 98%View Details
444731-52-6 -
 444731-52-6 98%View Details
444731-52-6 98%View Details
444731-52-6 -
 Pazopanib 98% CAS 444731-52-6View Details
Pazopanib 98% CAS 444731-52-6View Details
444731-52-6 -
 Pazopanib CAS 444731-52-6View Details
Pazopanib CAS 444731-52-6View Details
444731-52-6 -
 Pazopanib 99.94%View Details
Pazopanib 99.94%View Details
444731-52-6
