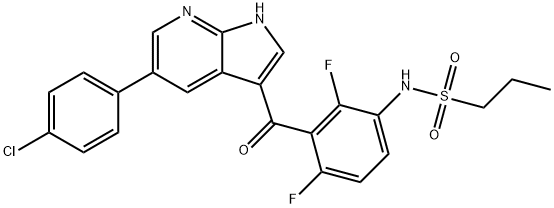
PLX4032
- CAS NO.:918504-65-1
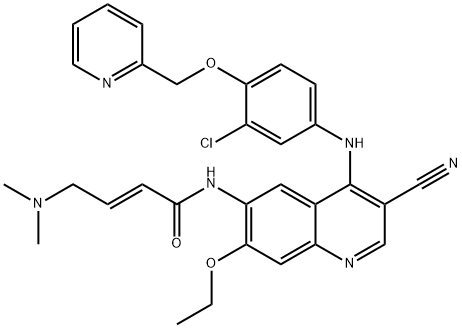
- Empirical Formula: C23H18ClF2N3O3S
- Molecular Weight: 489.92
- MDL number: MFCD18074504
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
What is PLX4032?
Absorption
Vemurafenib is well absorbed after oral administration. Peak concentrations are reached in 3 hours when an oral dose of 960 mg twice daily for 15 days has been given to patients. In the same conditions, Vemurafenib presents a Cmax of 62 mcg/ml and AUC of 601 mcg h/ml. It is unknown how food affects the absorption of vemurafenib. It presents an accumulation ratio of 7.36 after repeating doses of 960 mg
Toxicity
In the few toxicity reports, it has been shown an increased in the development of cutaneous squamous cell carcinomas or acceleration in pre-existant tumor growth.
Description
In August 2011, the United States FDA approved vemurafenib (PLX- 4032, RO-5185426) for the treatment of patients with metastatic melanoma with the BRAFV600E mutation. Vemurafenib has been developed as a targeted therapy for patients with the BRAF gene mutation since oncogenic B-raf signaling is implicated in approximately 50% of melanomas. Vemurafenib was identified based on an initial high-throughput screen followed by the extensive use of structure-based drug design. Vemurafenib is a potent inhibitor of B-RafV600E kinase (IC50=13 nM) compared to its potency against wildtype B-raf (IC50=160 nM) and is fairly selective versus a panel of 200 kinases. It does inhibit other kinases (RAF1, SRMS, ACK1, MAP4K5, and FGR) and mutant B-raf kinases (BRAFV600K, BRAFV600D, and BRAFV600R) with enzyme IC50's of <100 nM.
Chemical properties
Off-White Solid
Originator
Plexxikon (United States)
The Uses of PLX4032
Vemurafenib (PLX4032, RG7204) is a novel and potent inhibitor of B-RafV600E with IC50 of 31 nM
The Uses of PLX4032
Vemurafenib selective BRAFV600E kinase inhibitor; an antitumor agent. Vemurafenib functions by inhibiting the proliferation and mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase and ERK phosphorylation in a panel of tumor cell lines, including melanoma cell lines expressing BRAFV600E or other mutant BRAF proteins altered at codon 600.
The Uses of PLX4032
PLX4032 is an orally bioavailable, ATP-competitive inhibitor of mutant V600E and wild type B-Raf kinases (IC50s = 31 and 100 nM, respectively). It inhibits cell proliferation in a variety of cell lines expressing B-Raf V600E and synergizes strongly with taxol, vinblastine, and oxaliplatin in inhibiting the proliferation of B-RafV600E transformed cancer cells. PLX4032 is effective against the growth of tumors bearing the B-Raf V600E mutation.[Cayman Chemical]
What are the applications of Application
Vemurafenib is a novel and orally bioavailable ATP-competitive inhibitor of B-RAFV600E, potent and with an IC50 of 31 nM.
Indications
Vemurafenib is approved since 2011 for the treatment of metastatic melanoma with a mutation on BRAF in the valine located in the exon 15 at codon 600, this mutation is denominated as V600E. The V600E mutation, a substitution of glutamic acid for valine, accounts for 54% of the cases of cutaneous melanoma. Vemurafenib approval was extended in 2017, for its use as a treatment of adult patients with Erdheim-Chester Disease whose cancer cells present BRAF V600 mutation. Erdheim-Chester disease is an extremely rare histiocyte cell disorder that affects large bones, large vessels, central nervous system, as well as, skin and lungs. It is reported an association of Erdheim-Chester disease and V600E mutation.
Background
Vemurafenib is a competitive kinase inhibitor with activity against BRAF kinase with mutations like V600E. It exerts its function by binding to the ATP-binding domain of the mutant BRAF. Vemurafenib was co-developed by Roche and Plexxikon and it obtained its FDA approval on August 17, 2011, under the company Hoffmann La Roche. After approval, Roche in collaboration with Genentech launched a broad development program.
Definition
ChEBI: A pyrrolopyridine that is 1H-pyrrolo[2,3-b]pyridine which is substituted at position 5 by a p-chlorophenyl group and at positions 3 by a 3-amino-2,6-difluorobenzoyl group, the amino group of which has undergone ormal condensation with propane-1-sulfonic acid to give the corresponding sulfonamide. An inhibitor of BRAF and other kinases.
brand name
Zelboraf
Biological Activity
Vemurafenib (PLX4032, RG7204, RO5185426) is a novel and potent inhibitor of B-RafV600E with IC50 of 31 nM in cell-free assay. 10-fold selective for B-RafV600E over wild-type B-Raf in enzymatic assays and the cellular selectivity can exceed 100-fold. Vemurafenib (PLX4032, RG7204) induces autophagy.
Pharmacokinetics
BRAF activation results in cell growth, proliferation, and metastasis. BRAF is an intermediary molecule in MAPK whose activation depends on ERK activation, elevation of cyclin D1 and cellular proliferation. The mutation V600E produces a constitutively form of BRAF. Vemurafenib has been shown to reduce all activation markers related to BRAF; in clinical trials, vemurafenib treatment showed a reduction of cytoplasmic phosphorylated ERK and a cell proliferation driven by Ki-67. Studies also reported decrease in MAPK-related metabolic activity. All the different reports indicate thet Vemurafenib generates an almost complete inhibition of the MAPK pathway.
in vitro
PLX4032 inhibits B-RAFV600E, C-RAF, as well as wildtype B-RAF, with IC50 of 31 nM, 48 nM and 100 nM, respectively. PLX4032 also inhibits several non-RAF kinases, including ACK1, KHS1, and SRMS, with IC50 of 18 nM to 51 nM. In melanoma cell lines, the inhibitory effect by PLX4032 depends on B-RAF mutational status, because PLX4032 potently inhibits those harboring B-RAF V600 mutants, including V600E, V600D, V600K, and V600R, but not wildtype or other mutants. The IC50 values of PLX4032 on these cells, including MALME-3M, Colo829, Colo38, A375, SK-MEL28, and A2058, ranges from 20 nM to 1 μM. In these cells, PLX4032 (0.1 μM to 30 μM) also inhibits the phosphorylation of both MEK1/2 and ERK1/2. PLX4032 is highly effective in the treatment of melanoma, for its ability of inhibiting B-RAFV600E. However, PLX4032 displays limited effect in colon cancer patients that also carrying B-RAFV600E oncoprotein. The reason for this is that, in colon cancer cells, B-RAFV600E inhibition by PLX4032 results in a rapid feedback EGFR activation, which compensates for the PLX4032-inhibited cell proliferation.
in vivo
In B-RAFV600E-mutant mice xenograft models, PLX4032 (6 mg/kg–20 mg/kg) inhibits tumor growth. In mice xenograft models of LOX, Colo829, and A375 cells, PLX4032 (12.5 mg/kg–100 mg/kg) inhibits tumor growth and prolongs mice survival.
Metabolism
Vemurafenib is metabolized by CYP3A4 and the metabolites make up 5% of the components in plasma. The parent compound makes up for the remaining 95%.
Storage
Store at -20°C
References
1) Khazak?et al. (2007),?Selective Raf inhibition in cancer therapy; Expert Opin. Ther. Targets,?11?1587 2) Tap?et al.?(2010),?Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma; Neoplasia,?12?637 3) Lee?et al.?(2010),?PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas; Pigment Cell Melanoma Res.,?23?820 4) Flaherty?et al.?(2010),?Inhibition of mutated, activated BRAF in metastatic melanoma; N. Engl. J. Med.,?363?809
Properties of PLX4032
| Density | 1.46 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 100 mg/ml) |
| pka | 6.26±0.10(Predicted) |
| form | White powder. |
| color | White or off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 918504-65-1 |
Safety information for PLX4032
Computed Descriptors for PLX4032
PLX4032 manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Vemurafenib 99% (HPLC) CAS 918504-65-1View Details
Vemurafenib 99% (HPLC) CAS 918504-65-1View Details
918504-65-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
