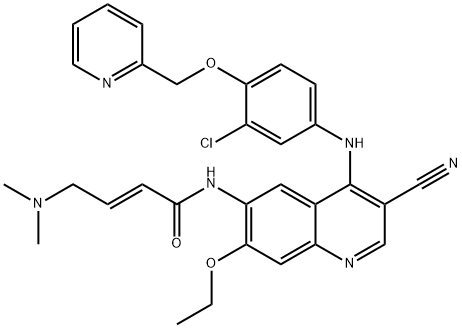
Nintedanib
- CAS NO.:656247-17-5
- Empirical Formula: C31H33N5O4
- Molecular Weight: 539.64
- MDL number: MFCD11974012
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Nintedanib?
Absorption
The absolute bioavailability of nintedanib is low at approximately 4.7%, likely owing to substantial first-pass metabolism and the effects of p-glycoprotein (P-gp) transporters. Tmax following oral administration is reached after approximately 2 hours in fasted patients and approximately 4 hours in fed patients, regardless of the food consumed. Administration of nintedanib following a high-fat, high-calorie meal resulted in an increase in Cmax by approximately 15% and an increase in AUC by approximately 20%. Age, body weight, and smoking status have been found to alter exposure to nintedanib, but these effects are not significant enough to warrant dose alterations.
Toxicity
Experience with nintedanib overdose is limited, but patients who inadvertently received higher-than-intended doses during initial trials experienced adverse effects consistent with the known safety profile of nintedanib, for example elevated liver enzymes and significant gastrointestinal effects.
There are no specific guidelines for the treatment of nintedanib overdose - in this case, therapy should be interrupted and general supportive measures employed as indicated.
The Uses of Nintedanib
It is an indolinone derivative potently blocking VEGF-, PDGF-, and FGF-receptor kinases; an indolinone as triple angiokinase inhibitors.
The Uses of Nintedanib
BIBF1120 (Vargatef) is a potent inhibitor of VEGFR1/2/3, FGFR1/2/3 and PDGFRα/β with IC50 of 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM and 59 nM/65 nM, respectively.
The Uses of Nintedanib
It is an indolinone derivative potently blocking VEGF-, PDGF-, and FGF-receptor kinases; an indolinone as triple angiokinase inhibitors. An antitumor agent.
What are the applications of Application
BIBF1120 is BIBF1120 is a potent kinase inhibitor found to affect VEGF, PDGF, and FGF receptors
Indications
Nintedanib is indicated for the treatment of idiopathic pulmonary fibrosis (IPF) and to slow declining pulmonary function in patients with systemic sclerosis-associated interstitial lung disease. It is also indicated for the treatment of chronic fibrosing interstitial lung diseases with a progressive phenotype.
In the EU, under the brand name Vargatef, nintedanib is indicated in combination with docetaxel for the treatment of adult patients with metastatic, locally advanced, or locally recurrent non-small cell lung cancer of adenocarcinoma histology who have already tried first-line therapy.
Background
Nintedanib is a small molecule kinase inhibitor used in the treatment of pulmonary fibrosis, systemic sclerosis-associated interstitial lung disease, and non-small cell lung cancer (NSCLC). It was first approved for use in the United States in 2014. Within the spectrum of idiopathic pulmonary fibrosis treatment options, nintedanib is currently one of only two disease-modifying therapies available and indicated for the condition (the other being Pirfenidone) and as such is used as a first-line treatment following diagnosis to slow down the progressive loss of lung function. As a chemotherapeutic agent for NSCLC, nintedanib, in combination with Docetaxel, is reserved for patients who have tried and failed first-line chemotherapeutic options.
Definition
ChEBI: A member of the class of oxindoles that is a kinase inhibitor used (in the form of its ethylsulfonate salt) for the treatment of idiopathic pulmonary fibrosis and cancer.
Biological Activity
nintedanib (bibf 1120) is an indolinone-derived oral active, triple angiokinase inhibitor of vascular endothelial growth factor receptor (vegfr)1-3, fibroblast growth factor receptor (fgfr)1-3 and platelet-derived growth factor receptor (pdgfr)α/β1. it has shown potent antiangiogenic activity at nanomolar (ic50, 20-100 nmol/l) by blocking these receptor-mediated signaling pathways1,2. nintedanib (bibf 1120) is in clinical development for the treatment of idiopathic pulmonary fibrosis as these receptors have been shown to be potentially involved in the pathogenesis of pulmonary fibrosis3,4. as a novel angiogenesis inhibitor, it is also being widely evaluated in different cancer models and has displayed significant anti-tumor activities by inhibiting tumor blood vessel formation5-7.to further evaluate its antitumor effects on multiple tumors, nintedanib is currently entering several
Pharmacokinetics
Nintedanib is a small molecule kinase inhibitor that inhibits upstream kinase activity to ultimately inhibit lung fibroblast proliferation and migration, as well as signalling pathways that promote the proliferation and survival of endothelial and perivascular cells in tumour tissues.
Nintedanib poses a risk of drug-induced liver injury, especially within the first three months of therapy. Liver function tests should be conducted at baseline prior to beginning therapy, at regular intervals for the first three months of therapy, and as indicated thereafter in patients exhibiting symptoms of hepatic injury such as jaundice or right upper quadrant pain. It is not recommended to be used in patients with pre-existing moderate to severe hepatic impairment (Child Pugh class B or C).
Clinical Use
Tyrosine kinase inhibitor:
Treatment of locally advanced, metastatic or locally
recurrent non-small cell lung cancer (NSCLC) of
adenocarcinoma tumour histology in combination
with docetaxel
Treatment of Idiopathic Pulmonary Fibrosis (IPF)
Side Effects
The most common side effects of Nintedanib in clinical studies were gastrointestinal-related side effects, including nausea (31%), vomiting (23%), diarrhoea (76%), abdominal pain (10%) and decreased appetite (10%). Other side effects were hepatic impairment commonly seen as elevated liver enzymes, weight loss (7 per cent), nasopharyngitis (7 per cent), cough (14 per cent), upper respiratory tract infections (14 per cent), headache (12 per cent), fatigue (14 per cent), skin ulcers (16 per cent) and urinary tract infections (12 per cent).
Reported post-marketing adverse events include gastrointestinal perforation, proteinuria and pancreatitis. In several clinical trials, nidanib has also been found to be associated with an increased risk of bleeding, possibly secondary to VEGFR inhibition. Although serious bleeding and arterial thromboembolic events (e.g., myocardial infarction (MI) and stroke) are rare, bleeding events with serious and fatal consequences have been reported in post-marketing surveillance. Therefore, the use of nidazanib has been associated with an increased risk of bleeding, possibly secondary to VEGFR inhibition. Therefore, patients treated with nidazanib and receiving full anticoagulation therapy need to be closely monitored for adverse bleeding events.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antifungals: concentration increased by
ketoconazole.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Metabolism
Nintedanib is predominantly metabolized via hydrolytic cleavage by esterases to its principle metabolite, BIBF 1202, which then undergoes glucuronidation via UGT enzymes in the intestines and liver (specifically UGT 1A1, UGT 1A7, UGT 1A8, and UGT 1A10) to form BIBF 1202 glucuronide. The CYP450 enzyme system plays a minor role in nintedanib metabolism, with CYP3A4 believed to be the main contributor - the major CYP-dependent metabolite of nintedanib, a demethylated metabolite termed BIBF 1053, could not be detected in plasma during pharmacokinetic studies and was found only in small quantities in the feces (approximately 4% of total dose). CYP-dependent metabolism of nintedanib accounts for roughly 5% of total drug metabolism, as opposed to 25% for esterase cleavage. Other minor metabolites, M7 and M8, are found in very small quantities in the urine (0.03% and 0.01%, respectively), though their origin and relevance is currently unclear.
Metabolism
Nintedanib is metabolised in the liver, initially by hydrolytic cleavage by esterases and then by glucuronidation by uridine diphosphate glucuronosyltransferase enzymes. Only a minor portion is metabolised by cytochrome P450 isoenzymes, mainly CYP3A4. More than 90% of a dose is eliminated by faecal/biliary excretion.
Storage
Store at -20°C
References
[1]hilberg, f. et al. bibf 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. cancer research 68, 4774-4782, doi:10.1158/0008-5472.can-07-6307 (2008).
Properties of Nintedanib
| Melting point: | >237oC (dec.) |
| Boiling point: | 742.2±60.0 °C(Predicted) |
| Density | 1.284±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 11.06±0.20(Predicted) |
| form | Yellow solid. |
| color | Light Yellow to Light Green |
Safety information for Nintedanib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Nintedanib
| InChIKey | XZXHXSATPCNXJR-ZIADKAODSA-N |
| SMILES | C(/C1C=CC=CC=1)(=C1\C(NC2=CC(C(=O)OC)=CC=C\12)=O)\NC1C=CC(N(C)C(=O)CN2CCN(C)CC2)=CC=1 |
Nintedanib manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 656247-17-5 Methyl 2-hydroxy-3- N-[4-[methyl-[2-(4-methyIpiperazin- 99%View Details
656247-17-5 Methyl 2-hydroxy-3- N-[4-[methyl-[2-(4-methyIpiperazin- 99%View Details
656247-17-5 -
 Nintedanib 98%View Details
Nintedanib 98%View Details -
 Nintedanib 98%View Details
Nintedanib 98%View Details
656247-17-5 -
 Nintedanib 656247-17-5 98%View Details
Nintedanib 656247-17-5 98%View Details
656247-17-5 -
 656247-17-5 Nintedanib 99%View Details
656247-17-5 Nintedanib 99%View Details
656247-17-5 -
 656247-17-5 98%View Details
656247-17-5 98%View Details
656247-17-5 -
 Vargatef 98% (HPLC) CAS 656247-17-5View Details
Vargatef 98% (HPLC) CAS 656247-17-5View Details
656247-17-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0