Acalabrutinib
- CAS NO.:1420477-60-6
- Empirical Formula: C26H23N7O2
- Molecular Weight: 465.51
- MDL number: MFCD29472294
- EINECS: 814-272-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-12 15:32:36
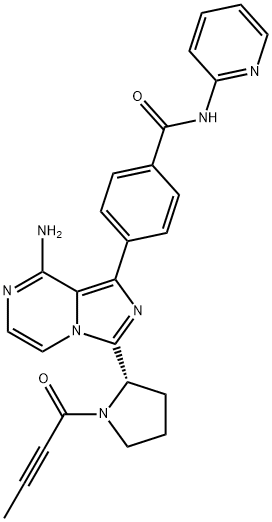
What is Acalabrutinib?
Absorption
The geometric mean absolute bioavailability of acalabrutinib is 25% with a median time to peak plasma concentrations (Tmax) of 0.75 hours.
Toxicity
Data regarding the toxicity of acalabrutinib is not readily available.
Description
Acalabrutinib (ACP-196) is a selective second-generation Bruton's tyrosine kinase (BTK) inhibitor with an IC50 of 3 nM, which prevents the activation of the B-cell antigen receptor (BCR) signaling pathway. ACP-196 has improved target specificity over ibrutinib with 323-, 94-, 19- and 9-fold selectivity over the other TEC kinase family members (ITK, TXK, BMX, and TEC, respectively) and no activity against EGFR.
The Uses of Acalabrutinib
Acalabrutinib, is an experimental anti-cancer drug and a selective Bruton's tyrosine kinase (BTK) inhibitor. This kinase transmits signals from B-cell Receptor (BCR), and thus any genetic BTK mutation causes B-Cell immunodeficiency. Therefore, BTK inhibitors targeting B-cell signaling has shown great promise for the treatment of chronic lymphocytic leukemia (CLL).
Background
To date, acalabrutinib has been used in trials studying the treatment of B-All, myelofibrosis, ovarian cancer, multiple myeloma, and Hodgkin lymphoma, among others.
As of October 31, 2017 the FDA approved Astra Zeneca's orally administered Calquence (acalabrutinib, capsules). This Bruton tyrosine kinase (BTK) inhibitor indicated for the treatment of chronic lymphocytic leukemia, small lymphocytic lymphoma, and in adult patients with Mantle cell lymphoma (MCL) who have already received at least one prior therapy. In August 2022, the FDA approved a new tablet formulation of Calquence, enabling the co-administration of this drug with proton pump inhibitors (PPIs). Unlike Calquence capsules, the co-administration of Calquence tablets and PPIs does not have an effect in the pharmacokinetics of acalabrutinib.
Also known as ACP-196, acalabrutinib is also considered a second generation BTK inhibitor because it was rationally designed to be more potent and selective than ibrutinib, theoretically expected to demonstrate fewer adverse effects owing to minimized bystander effects on targets other than BTK.
Nevertheless, acalabrutinib was approved under the FDA's accelerated approval pathway, which is based upon overall response rate and faciliates earlier approval of medicines that treat serious conditions or/and that fill an unmet medical need based on a surrogate endpoint. Continued approval for acalabrutinib's currently accepted indication may subsequently be contingent upon ongoing verification and description of clinical benefit in confimatory trials.
Furthermore, the FDA granted this medication Priority Review and Breakthrough Therapy designations. It also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases. At this time, more than 35 clinical trials across 40 countries with more than 2500 patients are underway or have been completed with regards to further research into better understanding and expanding the therapeutic uses of acalabrutinib .
Indications
Acalabrutinib is currently indicated for the treatment of adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. It has also been recently approved for chronic lymphocytic leukemia and small lymphocytic lymphoma.
Definition
ChEBI: Acalabrutinib is a member of the class of imidazopyrazines that is imidazo[1,5-a]pyrazine substituted by 4-(pyridin-2-ylcarbamoyl)phenyl, (2S)-1-(but-2-ynoyl)pyrrolidin-2-yl, and amino groups at positions 1, 3 and 8, respectively. It is an irreversible second-generation Bruton's tyrosine kinase (BTK) inhibitor that is approved by the FDA for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a secondary carboxamide, a member of benzamides, a member of pyridines, an aromatic amine, a pyrrolidinecarboxamide, an imidazopyrazine, a ynone and a tertiary carboxamide.
General Description
Class: non-receptor tyrosine kinase
Treatment: CLL, SLL, MCL
Oral bioavailability = 25%
Elimination half-life = 0.9 h
Protein binding = 97.5%
Pharmacokinetics
Acalabrutinib is a Bruton Tyrosine Kinase inhibitor that prevents the proliferation, trafficking, chemotaxis, and adhesion of B cells. It is taken every 12 hours and can cause other effects such as atrial fibrillation, other malignancies, cytopenia, hemorrhage, and infection.
in vitro
In the in vitro signaling assay on primary human CLL cells, acalabrutinib inhibits tyrosine phosphorylation of downstream targets of ERK, IKB, and AKT. Acalabrutinib demonstrates higher selectivity for BTK with IC50 determinations on nine kinases with a cysteine residue in the same position as BTK. Importantly, unlike ibrutinib, acalabrutinib does not inhibit EGFR, ITK, or TEC. acalabrutinib has no effect on EGFR phosphorylation on tyrosine residues Y1068 and Y1173. Compared with ibrutinib, acalabrutinib has much higher IC50(>1000 nM) or virtually no inhibition on kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1.
in vivo
Oral administration of ACP-196 in mice results in dose-dependent inhibition of anti-IgM-induced CD86 expression in CD19+ splenocytes with an ED50 of 0.34 mg/kg compared to 0.91 mg/kg for ibrutinib. A similar model is used to compare the duration of Btk inhibition after a single oral dose of 25 mg/kg. ACP-196 inhibits CD86 expression >90% at 3h postdose.
Metabolism
Acalabrutinib is mainly metabolized by CYP3A enzymes. ACP-5862 is identified to be the major active metabolite in plasma with a geometric mean exposure (AUC) that is about 2-3 times greater than the exposure of acalabrutinib. ACP-5862 is about 50% less potent than acalabrutinib in regards to the inhibition of BTK.
Metabolism
Acalabrutinib has an absolute bioavailability of 25% and an elimination half-life of 0.9 h. Because of its better oral bioavailability but much shorter half-life than ibrutinib, acalabrutinib is taken at a lower dosage (100 mg) than ibrutinib (420 mg) but twice a day (vs. QD for ibrutinib).
The major circulating metabolite in the plasma is the pyrrolidine ring-opened product 1. This metabolite also exhibited covalent BTK inhibition with potency 2-fold lower than acalabrutinib, and its kinase selectivity profile is similar to acalabrutinib. This active metabolite has a half-life of 6.9 h, much longer than the parent drug. However, the extent of its contribution to on-target covalent inhibition of BTK in humans remains to be established.
References
1) Wu et al.?(2016),?Acalabrutinib (ACP-196): a second-generation BTK inhibitor;?J. Hematol. Oncol.?9?21 2) Barf?et al.?(2017),?Acalabrutinib (ACP-196): A covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile;?J. Pharmacol. Exp. Ther.?363?240 3) Herman?et al.?(2017),?The Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia;?Clin. Cancer Res.?23?2831 4) Weber et al.?(2017),?Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity; Front. Immunol.?8?1454
Properties of Acalabrutinib
| Melting point: | >133°C (dec.) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Soluble in DMSO (up to at least 25 mg/ml) |
| form | solid |
| pka | 11.47±0.70(Predicted) |
| color | Yellow |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Acalabrutinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acalabrutinib
Acalabrutinib manufacturer
Salvavidas Pharmaceutical Pvt., Ltd.
Vijaya Pharma And Life Science
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1420477-60-6 Acalabrutinib 98%View Details
1420477-60-6 Acalabrutinib 98%View Details
1420477-60-6 -
 1420477-60-6 98%View Details
1420477-60-6 98%View Details
1420477-60-6 -
 Acalabrutinib 98%View Details
Acalabrutinib 98%View Details
1420477-60-6 -
 Acalabrutinib 1420477-60-6 98%View Details
Acalabrutinib 1420477-60-6 98%View Details
1420477-60-6 -
 Acalabrutinib 95% CAS 1420477-60-6View Details
Acalabrutinib 95% CAS 1420477-60-6View Details
1420477-60-6 -
 Acalabrutinib 98.00% CAS 1420477-60-6View Details
Acalabrutinib 98.00% CAS 1420477-60-6View Details
1420477-60-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1