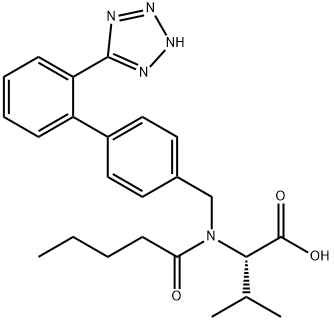
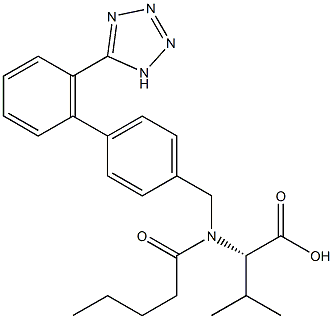
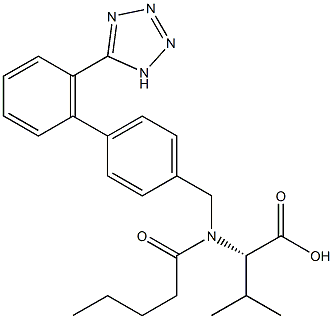
Valsartan
Synonym(s):N-(1-Oxopentyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L -valine;Valsartan
- CAS NO.:137862-53-4
- Empirical Formula: C24H29N5O3
- Molecular Weight: 435.52
- MDL number: MFCD00865840
- EINECS: 604-045-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Valsartan?
Absorption
After one oral dose, the antihypertensive activity of valsartan begins within approximately 2 hours and peaks within 4-6 hours in most patients. Food decreases the exposure to orally administered valsartan by approximately 40% and peak plasma concentration by approximately 50%. AUC and Cmax values of valsartan generally increase linearly with increasing dose over the therapeutic dose range. Valsartan does not accumulate appreciably in plasma following repetitive administration.
Toxicity
Approximate LD50 >2000 mg/kg (Gavage, rat)
Reproductive Toxicology Studies
No teratogenic effects were seen when valsartan was given to pregnant mice and rats at oral doses up to 600 mg/kg/day and to pregnant rabbits at oral doses reaching up to 10 mg/kg/day. Despite this, marked decreases in fetal weight, pup birth weight, pup survival rate, and delays in developmental milestones were noted in studies in which parental rats were treated with valsartan at oral, maternally toxic doses of 600 mg/kg/day during the organogenesis period or during late gestation and lactation.
Pregnancy
When used in pregnancy, drugs that act directly on the renin-angiotensin system (RAAS) can cause injury and death to the developing fetus. When pregnancy is detected, valsartan should be discontinued as soon as possible.
Description
Diovan(Valsartan) was launched in Germany and the UK as an angiotensin Ⅱ antagonist for use as an antihypertensive agent. Biphenylbromomethyl nitrite serves as the starting material for a three step synthesis of the compound, in which the (S)- enantiomer is more active than the (R)-enantiomer. Valsartan is a nonpeptide drug which is a highly specific antagonist of the AT1 receptor and is potent and orally active. This receptor is responsible for angiotensin Ⅱ cardiovascular effects (aldosterone and catecholamine secretion, vascular constriction, positive inotropic response and renal effects). Unlike losartan, it is not a prodrug and a single daily dose is comparible in activity to the ACE drug enalapril. It also did not exhibit the coughing side effect observed with ACE inhibitors. Diovan(Valsartan) is slowly metabolized (long lasting) with its main metabolite being significantly less active. There was no evidence of rebound hypertension when drug treatment was terminated and was as effective as the dihydropyridine Ca antagonist anlodipine.
Chemical properties
White Crystalline Powder
Originator
Norvartis (Switzerland)
The Uses of Valsartan
Valsartan is used as a first-line drug for the treatment of uncomplicated hypertension, isolated systolic hypertension, and left ventricular hypertrophy. Valsartan is a clinically widely used antihypertensive agent with the advantages of low side effects and good tolerability, and can also be used for the treatment of hypertension in patients with diabetes and renal disease.
Background
Valsartan is a member of the angiotensin II receptor blocker (ARB) class of drugs, alongside telmisartan, candesartan, losartan, olmesartan, and irbesartan. It works by selectively binding to the angiotensin receptor 1 (AT1), preventing the hypertensive effects of angiotensin II, such as vasoconstriction and aldosterone synthesis. This results in reduced blood pressure, lower aldosterone levels, decreased cardiac activity, and increased sodium excretion. Valsartan also impacts the renin-angiotensin aldosterone system (RAAS), which is vital for homeostasis and the regulation of kidney, vascular, and cardiac functions. Its blockade can help prevent cardiovascular and renal diseases and improve heart failure symptoms by reducing fluid retention and preventing ventricular hypertrophy. Initially approved in Europe in 1996 and in the U.S. in 1997, valsartan is well-tolerated with a favorable side-effect profile compared to other antihypertensive medications.
Indications
Valsartan is indicated for the treatment of hypertension to reduce the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. It is also indicated for the treatment of heart failure (NYHA class II-IV) and for left ventricular dysfunction or failure after myocardial infarction when the use of an angiotensin-converting enzyme inhibitor (ACEI) is not appropriate.
It is also used in combination with sacubitril.
What are the applications of Application
Valsartan is a nonpeptide angiotensin II AT1 receptor antagonist
Definition
ChEBI: A monocarboxylic acid amide consisting of L-valine in which the amino hydrogens have been replaced by a pentanoyl and a [2'-(1H-tetrazol-5-yl)biphenyl]-4-yl]methyl group. It exhibits antihypertensive activity.
brand name
Diovan (Novartis).
Therapeutic Function
Antihypertensive
General Description
Valsartan, N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine (Diovan), likelosartan, possesses the acidic tetrazole system, which mostlikely plays a role, similar to that of the acidic groups of angiotensinII, in binding to the angiotensin II receptor. In addition,the biphenyl system that serves to separate the tetrazolefrom the aliphatic nitrogen is still present. In addition, there isa carboxylic acid side chain in the valine moiety that alsoserves to bind to the angiotensin II receptor.
Biochem/physiol Actions
Valsartan is an Angiotensin II type 1 (AT1) receptor antagonist and anti-hypertensive. Valsartan renders protection against heart attack and stroke resulting from abrupt increase in blood pressure. Valsartan reduces myocardial-infarction-related complications in heart attack survivors.
Pharmacokinetics
Valsartan effectively inhibits the pressor effects of angiotensin II, with 80 mg doses providing approximately 80% inhibition at peak and around 30% lasting for 24 hours. It also causes a 2- to 3-fold increase in plasma renin and a consequent rise in angiotensin II concentration due to the removal of negative feedback, with minimal impact on plasma aldosterone levels. Valsartan does not significantly affect total cholesterol, fasting triglycerides, fasting serum glucose, or uric acid levels.
Contraindications
While excessive hypotension is rare in uncomplicated hypertensive patients treated with valsartan alone, caution is advised in patients with an activated renin-angiotensin system, particularly those receiving high doses of diuretics. Heart failure patients on valsartan may experience a reduction in blood pressure, but symptomatic hypotension is usually manageable with proper dosing and medical supervision. In cases of excessive hypotension, patients should be placed in the supine position and may require an intravenous infusion of normal saline. Once blood pressure stabilizes, treatment can typically continue without issues.
Patients with impaired renal function, such as those with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion, are at higher risk of acute renal failure when taking valsartan. Regular monitoring of renal function is necessary, and therapy may need to be withheld or discontinued if significant renal function decline occurs. Additionally, some heart failure patients on valsartan may experience hyperkalemia, particularly those with pre-existing renal impairment. This condition may require dosage adjustment or discontinuation of valsartan.
Synthesis
An aqueous solution (120 ml) of potassium hydroxide (0.568 mol, 31.8 g) is added in succession with 2-[(4-bromo-benzyl)-pentanoyl-amino]-3-methyl-butyric acid (0.811 mol, 30.0 g), tetrahydrofuran (120 ml), triphenylphosphine (0.0121 mol, 3.2 g) and palladium acetate (0.00405 mol, 0.91 g). The reaction mixture is refluxed and added with 2-(2H-tetrazol-5-yl)-benzene-boronic acid (0.142 mol, 27.0 g) in portions in about 6 h. After completion of the addition, the mixture is left to react for 2h, then cooled to room temperature and the phases are separated. The organic phase is diluted with water (120 ml) and tetrahydrofuran is distilled off under reduced pressure. The remaining aqueous solution is acidified to pH 6.5 and washed with isopropyl acetate (60 ml). The aqueous phase is acidified to pH 2 and diluted with isopropyl acetate (60 ml), the diphasic solution is filtered to remove phenyltetrazol. Phases are separated and the organic phase is concentrated under reduced pressure, to obtain a thick oil that is crystallized from isopropyl acetate (90 ml) and heptane (150 ml). The resulting product is filtered, washed twice with a 1:1 isopropyl acetate/heptane mixture (30 ml), and dried in static dryer at 45°C to obtain 28.2 g of Valsartan.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia,
hypotension and renal impairment with ACE-Is and
aliskiren.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Lithium: reduced excretion (possibility of enhanced
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity.
Side Effects
Common side effects of Valsartan include: dizziness, headache, feeling sick (nausea), vomiting, diarrhoea, joint or muscle pain. Serious side effects include: liver problems (yellowing of the skin and eyes), easy bruising or appearance, thrombocytopenia, weakness, irregular heartbeat, pins and needles, and muscle cramps. Serious allergic reactions are relatively rare. Long-term use of valsartan may lead to hyperkalaemia and kidney dysfunction. Contact your doctor if any serious adverse reactions occur.
Metabolism
Valsartan undergoes minimal liver metabolism and is not biotransformed to a high degree, as only approximately 20% of a single dose is recovered as metabolites. The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism.
Metabolism
Valsartan is not highly metabolised as only about 20% of dose is recovered as metabolites. A hydroxy metabolite has been identified in plasma at low concentrations (less than 10% of the valsartan AUC). This metabolite is pharmacologically inactive.
Valsartan is mainly eliminated by biliary excretion in faeces (about 83% of dose) and renally in urine (about 13% of dose), mainly as unchanged drug.
Storage
Store at RT
References
1) Criscione?et al. (1993),?Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype; Br. J. Pharmacol.,?110?761 2) Wexler?et al. (1996), Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy; J. Med. Chem.,?39?625 3) Iwashita?et al. (2013),?Valsartan restores inflammatory response by macrophages in adipose and hepatic tissues of LPS-infused mice; Adipocyte,?2?28
Properties of Valsartan
| Melting point: | 116-117°C |
| Boiling point: | 684.9±65.0 °C(Predicted) |
| Density | 1.212±0.06 g/cm3(Predicted) |
| vapor pressure | 0-0Pa at 20-25℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 3.56±0.10(Predicted) |
| form | powder |
| color | white to tan |
| optical activity | [α]/D -55 to -70°, c = 1 in methanol |
| Water Solubility | 84.99mg/L(25 ºC) |
| Merck | 14,9916 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 137862-53-4(CAS DataBase Reference) |
| EPA Substance Registry System | L-Valine, N-(1-oxopentyl)-N-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]- (137862-53-4) |
Safety information for Valsartan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Valsartan
| InChIKey | ACWBQPMHZXGDFX-QFIPXVFZSA-N |
Valsartan manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](https://img.chemicalbook.in/CAS/GIF/137863-20-8.gif)
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](https://img.chemicalbook.in/CAS/20150408/GIF/149709-59-1.gif)
You may like
-
 Valsartan - IP/USP 137862-53-4 98%View Details
Valsartan - IP/USP 137862-53-4 98%View Details
137862-53-4 -
 137862-53-4 98%View Details
137862-53-4 98%View Details
137862-53-4 -
 Valsartan 98%View Details
Valsartan 98%View Details -
 Valsartan 137862-53-4 98%View Details
Valsartan 137862-53-4 98%View Details
137862-53-4 -
 Valsartan 95% CAS 137862-53-4View Details
Valsartan 95% CAS 137862-53-4View Details
137862-53-4 -
![N-(1-Oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine 97% CAS 137862-53-4](https://img.chemicalbook.in//Content/image/CP5.jpg) N-(1-Oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine 97% CAS 137862-53-4View Details
N-(1-Oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine 97% CAS 137862-53-4View Details
137862-53-4 -
 Valsartan >98% (HPLC) CAS 137862-53-4View Details
Valsartan >98% (HPLC) CAS 137862-53-4View Details
137862-53-4 -
 Valsartan 98% (HPLC) CAS 137862-53-4View Details
Valsartan 98% (HPLC) CAS 137862-53-4View Details
137862-53-4
