Testosterone undecanoate
- CAS NO.:5949-44-0
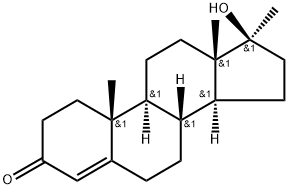
- Empirical Formula: C30H48O3
- Molecular Weight: 456.71
- MDL number: MFCD00468114
- EINECS: 227-712-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-24 17:44:56
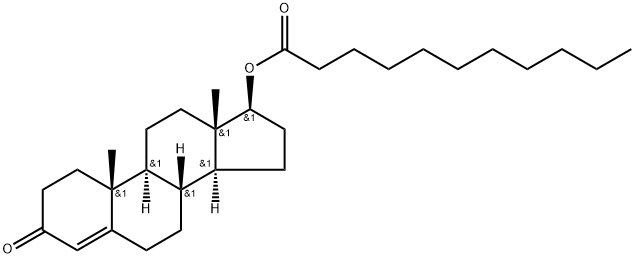
What is Testosterone undecanoate?
Absorption
Testosterone undecanoate is a lipophilic molecule that is absorbed into the intestinal lymphatic system after oral administration. It is then released into the general blood circulation by the thoracic duct, thereby bypassing the portal circulation and first-pass metabolism in the liver, unlike endogenous testosterone.
Following oral administration of 237 mg twice per day in males with hypogonadism, the mean (SD) Cmax was 1008 (581) ng/dL. Tmax is about five hours following oral administration. Decreased testosterone exposure was observed when administered without food.
Following intramuscular administration of 750 mg testosterone undecanoate, serum testosterone concentrations reached a maximum after a median of seven days (range of four to 42 days), which then slowly declined. The mean (SD) Cmax was about 90.9 (68.8) ng/dL on the fourth day following injection of testosterone undecanoate. Steady-state serum testosterone concentration was achieved with the third injection at 14 weeks. At 42 days following the injection, testosterone undecanoate was nearly undetectable.
Toxicity
The oral LD50 is 4000 mg/kg in mice and rats. The subcutaneous LD50 is 2880 mg/kg in mice and rats.
There is limited information on testosterone undecanoate overdose. There was one report of acute overdose from an approved injectable testosterone product, which resulted in increased serum testosterone levels of up to 11,400 ng/dL with a cerebrovascular accident. There was one case of overdose following administration of oral testosterone undecanoate capsules: this patient inadvertently took a 20% higher dose than the maximum recommended dose but did not report any adverse reactions. Overdose should be managed with discontinuation of the drug in combination with appropriate symptomatic and supportive care.
The abuse of anabolic androgenic steroids can result in serious adverse reactions, such as cardiac arrest, myocardial infarction, hypertrophic cardiomyopathy, congestive heart failure, cerebrovascular accident, hepatotoxicity, and psychiatric manifestations, including major depression, mania, paranoia, psychosis, delusions, hallucinations, hostility, and aggression. Men receiving testosterone have experienced transient ischemic attacks, convulsions, hypomania, irritability, dyslipidemias, testicular atrophy, subfertility, and infertility.
Description
Testosterone undecanoate, sold under the brand names Andriol and Aveed among others, is an androgen and anabolic steroid (AAS) medication that is used mainly in the treatment of low testosterone levels in men. which includes hormone therapy for transgender men. It is taken by mouth or given by injection into muscle.
Testosterone Undecanoate is the undecanoate ester form of the androgen testosterone, with gonadotropin-secretory inhibiting and hormone replacement activity. As testosterone inhibits the secretion of gonadotropins from the pituitary gland, administration of testosterone decreases the secretion of luteinizing hormone (LH). By inhibiting LH secretion, the growth of Leydig cells, which are normally stimulated by LH to produce testosterone, may be suppressed. In addition, this agent promotes the maintenance of male sex characteristics and can be used for testosterone replacement in hypogonadal males.
Chemical properties
White Solid
The Uses of Testosterone undecanoate
Testosterone undecanoate is the ester prodrug of testosterone and has a midchain fatty acid at the 17Beta position. It is available as an intramuscular injection and as an oral capsule, and is indicated for treatment of testosterone deficiency. It should be noted that testosterone undecanoate is only indicated for treatment of hypogonadal conditions with structural or genetic etiologies and should not be used to manage "age-related hypogonadism".
Indications
Testosterone undecanoate is indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone. These conditions include:
Testosterone undecanoate is not used to treat age-related hypogonadism.
Background
Testosterone undecanoate is the ester prodrug of testosterone and has a mid-chain fatty acid at the carbon 17β position. It was developed via fatty acid esterification of testosterone in order to achieve orally administer testosterone. There are oral and intramuscular formulations available for testosterone undecanoate: both formulations are indicated for testosterone replacement therapy in adult males with hypogonadism.
Testosterone is a critical male hormone that is responsible for the normal growth and development of the male sex organs and for the maintenance of secondary sex characteristics. Male hypogonadism, resulting from insufficient testosterone secretion, can result symptoms and signs of testosterone deficiency, such as decreased libido, erectile dysfunction, and loss of muscle and bone mass. Testosterone replacement therapy aims to restore the levels of testosterone, thereby improving symptoms and signs of testosterone deficiency.
Indications
JATENZO (testosterone undecanoate) is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
Primary hypogonadism (congenital or acquired): testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
www.accessdata.fda.gov
Definition
ChEBI: Testosterone undecanoate is a steroid ester. It is a metabolite of Testosterone. It is a promising androgen for male hormonal contraception.
Pharmacokinetics
Once in circulation, testosterone undecanoate is cleaved to release testosterone, which mediates a range of biological actions. Testosterone is an endogenous male hormone that plays a key role in male sexual differentiation: it is involved in the regulation of hematopoiesis, body composition, and bone metabolism. As a hormone replacement therapy, testosterone undecanoate is an exogenous source of testosterone in males with hypogonadism. Testosterone therapy aims to improve symptoms and signs of testosterone deficiency including decreased libido, erectile dysfunction, and loss of muscle and bone mass.
Testosterone has a controlled substance in the US due to the abuse potential by athletes and bodybuilders. The use of testosterone at higher doses than recommended can lead to withdrawal symptoms lasting for weeks or months. Withdrawal symptoms include depressed mood, major depression, fatigue, craving, restlessness, irritability, anorexia, insomnia, decreased libido, and hypogonadotropic hypogonadism.
Testosterone can cause hirsutism, virilization, deepening of the voice, clitoral enlargement, breast atrophy, male-pattern baldness, and menstrual irregularities when administered to women. The use in adolescents can lead to the premature closure of bony epiphyses with termination of growth and precocious puberty.
Pharmacokinetics
Testosterone undecanoate is a prodrug of testos-terone esterified in the 17b-position with undecanoic acid. Following oral administration, thisprodrug is metabolized only partly in the intestinal wall, and the remaining fraction of testosterone undecanoate is absorbed via the lymphatic system, and hence, avoids first pass hepatic metabolism. After reaching the systemic circulation, testosterone undecanoate rapidly converts to free testosterone. The absolute bioavailability of testosterone following oral administration of testosterone undecanoate is estimated to be 7%. Recent studies in lymph duct cannulated dogs revealed that all of the testosterone undecanoate absorbed into the systemic circulation is due to lymphatic absorption of the prodrug, and more than 80% of the free testosterone in the plasma is contributed by systemic hydrolysis of the lymphatically transported prodrug.
Side Effects
Side effects of testosterone undecanoate include symptoms of masculinization like acne, increased hair growth, voice changes, hypertension, elevated liver enzymes, hypertriglyceridemia, and increased sexual desire. The drug is a prodrug of testosterone, the biological ligand of the androgen receptor (AR) and hence is an androgen and anabolic steroid. It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy. Testosterone undecanoate is a testosterone ester and a prodrug of testosterone in the body. Because of this, it is considered to be a natural and bioidentical form of testosterone.
Metabolism
Testosterone undecanoate can be reduced to dihydrotestosterone undecanoate via 5α-reductase. In the circulation, the ester bond linking testosterone to the undecanoic acid is cleaved by endogenous non-specific esterases. Like all fatty acids, the undecanoic side chain undergoes β-oxidation to form acetyl coenzyme A (CoA) and, finally, propionyl CoA.
Testosterone is metabolized to various 17-keto steroids through two different pathways to form major active metabolites, estradiol and dihydrotestosterone (DHT).
Properties of Testosterone undecanoate
| Melting point: | 59-61°C |
| Boiling point: | 550.7±50.0 °C(Predicted) |
| Density | 1.03±0.1 g/cm3(Predicted) |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | DMSO : 10 mg/mL (21.90 mM; Need ultrasonic and warming) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 5949-44-0(CAS DataBase Reference) |
Safety information for Testosterone undecanoate
Computed Descriptors for Testosterone undecanoate
| InChIKey | UDSFVOAUHKGBEK-JCROKFDDNA-N |
| SMILES | C[C@]12CCC(=O)C=C1CC[C@@]1([H])[C@]3([H])CC[C@H](OC(=O)CCCCCCCCCC)[C@@]3(C)CC[C@]21[H] |&1:1,10,12,16,30,34,r| |
Testosterone undecanoate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 5949-44-0 Testosterone undecanoate 98%View Details
5949-44-0 Testosterone undecanoate 98%View Details
5949-44-0 -
 5949-44-0 Testosterone undecanoate 98%View Details
5949-44-0 Testosterone undecanoate 98%View Details
5949-44-0 -
 5949-44-0 98%View Details
5949-44-0 98%View Details
5949-44-0 -
 Cas 5949 44 0 Testosterone Undecanoate, IPView Details
Cas 5949 44 0 Testosterone Undecanoate, IPView Details
5949-44-0 -
 Testosterone Undecanoate API PowderView Details
Testosterone Undecanoate API PowderView Details
5949-44-0 -
 Testosign Testosterone Undecanoate 40mg Soft Gelatin Capsules, 3*10View Details
Testosign Testosterone Undecanoate 40mg Soft Gelatin Capsules, 3*10View Details
5949-44-0 -
 Grade: A Grade Testosterone Undecanoate 40 Mg tablets, For Treatment Of Male Hypogonadism, Purity: 90%View Details
Grade: A Grade Testosterone Undecanoate 40 Mg tablets, For Treatment Of Male Hypogonadism, Purity: 90%View Details
5949-44-0 -
 Cernos Depot Testosterone Undecanoate Injection 1000mg/4mlView Details
Cernos Depot Testosterone Undecanoate Injection 1000mg/4mlView Details
5949-44-0
