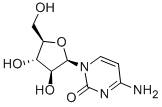
5-Azacytidine
Synonym(s):4-Amino-1-(β-D -ribofuranosyl)-1,3,5-triazin-2(1H)-one;5′AzaC, 5-Azacitidine, Zcyd, 4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one;5-Azacitidine;5-Azacytidine;Ladakamycin
- CAS NO.:320-67-2
- Empirical Formula: C8H12N4O5
- Molecular Weight: 244.2
- MDL number: MFCD00006539
- EINECS: 206-280-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
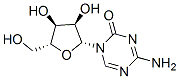
What is 5-Azacytidine?
Absorption
Azacitidine is rapidly absorbed after subcutaneous administration. In adult patients with myelodysplastic syndrome given a single subcutaneous dose of 75 mg/m2 of azacitidine, the Cmax and Tmax were 750 ng/ml and 0.5 hours, respectively. Based on the area under the curve, the bioavailability of subcutaneous azacitidine relative to intravenous azacitidine is approximately 89%. In 21 patients with cancer given subcutaneous azacitidine, the AUC and Cmax were approximately dose-proportional between 25 and 100 mg/m2. Multiple subcutaneous or intravenous doses of azacitidine are not expected to result in drug accumulation.
Toxicity
One case of overdose with azacitidine was reported during clinical trials. After receiving a single dose of 290 mg/m2 of azacitidine intravenously (almost 4 times the recommended starting dose), a patient experienced diarrhea, nausea, and vomiting. These adverse events resolved without sequelae, and the correct dose was resumed the following day. In case of overdose, patients should be monitored with appropriate blood counts and receive supportive treatment as necessary. There is no known specific antidote for azacitidine overdosage. In mice, the oral LD50 of azacitidine is 572 mg/kg, while the intravenous LD50 is approximately 117 mg/kg.
Description
5-Azacytidine is an analog of the nucleoside cytidine which can be incorporated into DNA and RNA. 5-Azacytidine acts as an epigenetic modifier by incorporating into DNA where it irreversibly binds to DNA methyltransferases, thus inhibiting their activity.
Azacitidine is an antineoplastic agent launched for the treatment of myelodysplastic syndrome (MDS). MDS is a group of closely related diseases caused by abnormal blood-forming stem cells of the bone marrow. Azacitidine is indicated for the treatment of all five subtypes of MDS, which consist of refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia.
Chemical properties
White crystalline solid or powder. Soluble in dimethyl sulfoxide, slightly soluble in ethanol:water (50:50), propylene glycol, polyethylene glycol; slightly soluble in water, saturated octanol aqueous solution, 5% glucose injection, N-methyl-2-pyrrolidone, 0.9% sodium chloride injection and 5% polysorbate 80 aqueous solution; insoluble in acetone, ethanol and methyl ethyl ketone.
Originator
Pharmion (US)
The Uses of 5-Azacytidine
5-Azacytidine, its incorporation into RNA alters RNA synthesis and processing, and results in inhibition of protein synthesis. It has been used as a cancer chemotherapeutic agent. It is a powerful bacteriostatic, antitumor, and mutagenic agent; it also exhibits immunosuppressive, antimitotic, radioprotective, and virostatic effects.
The Uses of 5-Azacytidine
A potent growth inhibitor and cytotoxic agent. It acts as a demethylating agent by inhibiting DNA methyltransferase
The Uses of 5-Azacytidine
Antineoplastic;'Antimetabolite
The Uses of 5-Azacytidine
antianginal
Indications
Azacitidine (for subcutaneous or intravenous use) is indicated for the treatment of adult patients with the following French-American-British (FAB) myelodysplastic syndrome (MDS) subtypes: refractory anemia (RA) or refractory anemia with ringed sideroblasts (RARS) (if accompanied by neutropenia or thrombocytopenia or requiring transfusions), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMMoL). Azacitidine is also indicated for the treatment of pediatric patients aged 1 month and older with newly diagnosed Juvenile Myelomonocytic Leukemia (JMML).
Azacitidine (for oral use) is indicated for continued treatment of adult patients with acute myeloid leukemia (AML) who achieved first complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy.
What are the applications of Application
5-Azacytidine is a potent growth and Dnmt inhibitor
Background
Azacitidine is a pyrimidine nucleoside analogue with anti-neoplastic activity. It differs from cytosine by the presence of nitrogen in the C5-position, key in its hypomethylating activity. Two main mechanisms of action have been proposed for azacitidine. One of them is the induction of cytotoxicity. As an analogue of cytidine, it is able to incorporate into RNA and DNA, disrupting RNA metabolism and inhibiting protein and DNA synthesis. The other one is through the inhibition of DNA methyltransferase, impairing DNA methylation. Due to its anti-neoplastic activity and its ability to inhibit methylation in replicating DNA, azacytidine has been used mainly used in the treatment of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), two types of cancer characterized by the presence of aberrant DNA methylation.
In May 2004, the FDA approved the use of azacitidine administered subcutaneously for the treatment of MDS of all French-American-British (FAB) subtypes. In January 2007, the FDA approved the intravenous administration of azacitidine. The use of oral azacitidine for the treatment of AML in patients in complete remission was approved by the FDA in September 2020.
Definition
ChEBI: A N-glycosyl-1,3,5-triazine that is 4-amino-1,3,5-triazin-2(1H)-one substituted by a beta-D-ribofuranosyl residue via a N-glycosidic linkage.
Manufacturing Process
A mixture of 1-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-4-methylthio-1,2-
dihydro-1,3,5-triazin-2-one (0.5875 g), absolute methanol (5 ml) and a
normal methanolic sodium methoxide solution (1.2 ml) is stirred at room
temperature with the exclusion of atmospheric moisture (a guard tube filled
with potassium hydroxide pellets is fitted to the reaction vessel). The starting
compound passes into solution in the course of 5 min. The resulting solution is
allowed to stand at room temperature for 45 min and then the cations are
removed by passage of the solution through a column packed with 10 ml of a
weakly acidic cation exchange resin in the H+ form prewashed with water and
methanol. The methanolic effluent (60 ml) is evaporated under reduced
pressure at 30°C, the residue is dissolved in methanol (20 ml) and the
solution once again is evaporated and the 1-β-D-ribofuranosyl-4-methoxy-1,2-
dihydro-1,3,5-triazin-2-one was obtained.
The residual crude crystalline 1β-D-ribofuranosyl-4-methoxy-1,2-dihydro-
1,3,5-triazin-2-one is dissolved in a 10% solution of dry ammonia in absolute
methanol (4 ml) and the whole reaction mixture is allowed to stand in a
stoppered flask for 30 min at room temperature (the product begins to
deposit in the course of 5 min) and for 12 h in a refrigerator at -10°C. The
resulting 5-azacytidine is collected with suction, washed with methanol and
dried under reduced pressure. A yield of 0.216 g (88.6%) of 5-azacytidine,
that is [1-β-D-ribofuranosyl-4-amino-1,3,5-triazin-2(1H)-one], melting point
232°-234°C (dec.), is obtained.
brand name
Vidaza (Pharmion).
Therapeutic Function
Antineoplastic
Biological Functions
Azacitidine is given subcutaneously for the treatment of myelodysplastic syndrome, and serum levels generally are maximized within 30 minutes. The parent drug and its metabolites are excreted in the urine. Azacitidine is carcinogenic and teratogenic in rodents, and leukopenia, thrombocytopenia, and neutropenia are the most common reasons for drug discontinuation or dosage reduction.
General Description
White crystalline powder.
Air & Water Reactions
Slightly water soluble. Unstable in solution.
Reactivity Profile
5-Azacytidine is sensitive to light (may discolor). 5-Azacytidine is sensitive to oxidation. 5-Azacytidine is unstable in solution. 5-Azacytidine undergoes hydrolysis in aqueous buffers. 5-Azacytidine is incompatible with strong oxidizers.
Fire Hazard
Flash point data for 5-Azacytidine are not available; however, 5-Azacytidine is probably combustible.
Biological Activity
DNA methyltransferase inhibitor. Incorporates into DNA forming covalent adducts with cellular DNMT1, depleting enzyme activity. Induces demethylation and reactivation of silenced genes. Improves the efficiency of reprogramming of stem cells.
Biochem/physiol Actions
5-Azacytidine is a deoxycytidine analog and a demethylating agent. It acts as a potential antineoplastic agent for acute myelogenous leukemia. 5-Azacytidine activates repressed genes by inhibiting DNA methylation. 5-Azacytidine also affects protein synthesis by altering the RNA function and stability.
Pharmacokinetics
The concentration of azacitidine required for maximum inhibition of DNA methylation in vitro does not cause major suppression of DNA synthesis, and hypomethylation may restore normal function to genes critical for differentiation and proliferation. Genome-wide DNA methylation levels in bone marrow granulocytes were reduced in patients with juvenile myelomonocytic leukemia after the first treatment cycle of azacitidine (75 mg/m2 or 2.5 mg/kg), confirming the DNA-hypomethylating activity of azacitidine.
The use of azacitidine causes anemia, neutropenia and thrombocytopenia in adult patients with myelodysplastic syndrome and pediatric patients with juvenile myelomonocytic leukemia. Azacitidine may cause renal toxicity, tumor lysis syndrome and embryo-fetal toxicity. It may also lead to the development of hepatotoxicity in patients with severe pre-existing hepatic impairment.
Clinical Use
Antineoplastic agent:
Treatment of people not eligible for stem cell
transplants with myelodysplastic syndromes,
chronic myelomonocytic leukaemia or acute myeloid
leukaemia
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic data. Poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by intravenous route: nausea, vomiting and dlarrhea, reduction in white cell count (luekopenia and agranulocytosis). An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin irritant. When heated to decomposition it emits toxic fumes of NOx.
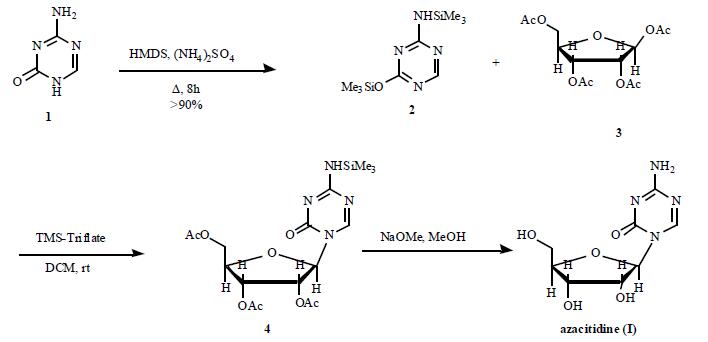
Synthesis
The triazine ring of azacitidine is sensitive to water; this characteristic has made the synthesis of azacitidine a challenge, especially in manufacturing at commercial scale. A number of reports have appeared in order to avoid the use of water; however, these methods all have additional problems that render them undesirable for the large scale synthesis. A recent improved synthesis is depicted in the Scheme. 5- Azacytosine (1) was bis-silylated with HMDS in the presence of (NH4)SO4 to furnish trimethylsilylated azacytosine (2) in greater than 90% yield. Coupling of silylated azacytosine 2 with 1,2,3,5-tetra-O-acetyl-b-Dribofuranose (3) in DCM in the presence of TMS-triflate provided protected 5-azacitidine 4. The acetyl groups were then removed by using NaOMe in MeOH at rt. The crude azacitidine was crystallized from DMSO/MeOH to provide pure azacitidine (I).
Potential Exposure
A growth inhibitor (DNA methyltransferase inhibitor). A cytotoxic agent and chemotherapeutic agent used to treat angina pectoris, an ischemic heart disease symptom. Occupational exposure to azacitidine could occur among health professionals and support staff (including custodians) by dermal contact, inhalation, or accidental ingestion during drug preparation or administration or cleanup of medical waste, including disposal of excretions from treated patients (Zimmerman et al. 1981, NIOSH 2004). The National Occupational Exposure Survey (conducted from 1981 to 1983) estimated that 1069 healthservices workers, including 698 women, potentially were exposed to azacitidine. Azacitidine may be produced synthetically or isolated from the bacterium Streptoverticillium ladakanus . Incompatibilities: Azacitidine is Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with alkali metals, nitrides, and strong reducing agents such as hydrides may form flammable and/or toxic gases. May react with anhydrides forming acids and esters, generating noticeable heat, and also with oxoacids and carboxylic acids to form esters plus water, but the heat of reaction in the latter case typically is low. Keep away from isocyanates and epoxides; may initiate their polymerization. Azacitidine is sensitive to light and oxidation and unstable in solution. It undergoes hydrolysis in aqueous buffers.
Carcinogenicity
Azacitidine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Metabolism
An in vitro study of azacitidine incubation in human liver fractions indicated that cytochrome P450 (CYP) enzymes do not participate in the metabolism of azacitidine. Azacitidine is metabolized through spontaneous hydrolysis and deamination mediated by cytidine deaminase.
Metabolism
Azacitidine undergoes spontaneous hydrolysis and deamination mediated by cytidine deaminase. Following IV administration of radioactive azacitidine to 5 cancer patients, the cumulative urinary excretion was 85% of the radioactive dose. Faecal excretion accounted for <1% of administered radioactivity over three days. Mean excretion of radioactivity in urine following SC administration of [14C]-azacitidine was 50%.
Storage
Room temperature
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Azacitidine is Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with alkali metals, nitrides, and strong reducing agents such as hydrides may form flammable and/or toxic gases. May react with anhydrides forming acids and esters, generating noticeable heat, and also with oxoacids and carboxylic acids to form esters plus water, but the heat of reaction in the latter case typically is low. Keep away from isocyanates and epoxides; may initiate their polymerization. Azacitidine is sensitive to light and oxidation and unstable in solution. It undergoes hydrolysis in aqueous buffers.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
References
1) Giraldo et al. (2011), Inhibition of DNA Methylation in somatic cells ; Methods Mol. Biol., 791 145 2) Brueckner et al. (2005), Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA Methyltransferases; Cancer Res., 65 6305 3) Mikkelsen et al. (2008), Dissecting direct reprogramming through integrative genomic analysis; Nature, 454 49 4) Qian et al. (2011), 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extra cellular regulated kinase; Stem Cells Dev., 21 67 5) Kiziltepe et al. (2007), 5-Azacytidine, a DNA Methyltranserase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells; Mol. Cancer Ther., 6 1718
Properties of 5-Azacytidine
| Melting point: | 226-232 °C (dec.)(lit.) |
| Boiling point: | 387.12°C (rough estimate) |
| alpha | 40 º (C=1, H2O 22 ºC) |
| Density | 1.4287 (rough estimate) |
| refractive index | 1.6590 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml), or in Water (up to 12 mg/ml). |
| form | lyophilized powder |
| pka | 13.46±0.70(Predicted) |
| color | White to Off-white |
| Water Solubility | 0.5-1.0 g/100 mL at 21 ºC |
| Merck | 14,887 |
| BRN | 620461 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 320-67-2(CAS DataBase Reference) |
| IARC | 2A (Vol. 50) 1990 |
| EPA Substance Registry System | Azacitidine (320-67-2) |
Safety information for 5-Azacytidine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H341:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 5-Azacytidine
| InChIKey | NMUSYJAQQFHJEW-WGDKFINWSA-N |
5-Azacytidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Azacitidine 98%View Details
Azacitidine 98%View Details -
 Azacitidine 98%View Details
Azacitidine 98%View Details
320-67-2 -
 5-Azacytidine 99%View Details
5-Azacytidine 99%View Details
320-67-2 -
 320-67-2 5-Azacytidine 98%View Details
320-67-2 5-Azacytidine 98%View Details
320-67-2 -
 320-67-2 95-99%View Details
320-67-2 95-99%View Details
320-67-2 -
 5-Azacytidine, ≥98% (HPLC) CAS 320-67-2View Details
5-Azacytidine, ≥98% (HPLC) CAS 320-67-2View Details
320-67-2 -
 5-Azacytidine CAS 320-67-2View Details
5-Azacytidine CAS 320-67-2View Details
320-67-2 -
 Azacitidine CAS 320-67-2View Details
Azacitidine CAS 320-67-2View Details
320-67-2
