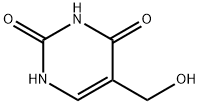
5-Hydroxymethyluracil
- CAS NO.:4433-40-3
- Empirical Formula: C5H6N2O3
- Molecular Weight: 142.11
- MDL number: MFCD00056024
- EINECS: 224-636-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is 5-Hydroxymethyluracil?
Description
5-Hydroxymethyluracil is a product of oxidative damage to DNA, predominantly by hydroxyl radical via the Fenton reaction. It can be used as a potential epigenetic mark enhancing or inhibiting transcription with bacterial RNA polymerase.
Chemical properties
White Powder
The Uses of 5-Hydroxymethyluracil
5-Hydroxymethyluracil (cas# 4433-40-3) is a compound useful in organic synthesis.
What are the applications of Application
Hydroxymethyl uracil is a stable major oxidative modification of thymine formed through the action of ionizing radiation
Definition
ChEBI: 5-hydroxymethyluracil is a primary alcohol that is uracil bearing a hydroxymethyl substituent at the 5-position. It has a role as a human metabolite. It is a primary alcohol and a pyrimidone. It derives from a uracil.
Biosynthesis
5-Hydroxymethyluracil is produced by the enzyme thymine dioxygenase (EC 1.14.11.6) which catalyzes the chemical reaction thymine + 2-oxoglutarate + O2 <-> 5-hydroxymethyluracil + succinate + CO2.
The 3 substrates of this enzyme are thymine, 2-oxoglutarate, and O2, whereas its 3 products are 5-hydroxymethyluracil, succinate, and CO2. The 5hmU base can also be generated by oxidation/hydroxylation of thymine by the Ten-Eleven-Translocation (TET) proteins or result from deamination of 5hmC. DNA containing 5hmU has been reported to be more flexible and hydrophilic.
Biotechnological Production
5-Hydroxymethyluracil (5hmU) is a thymine base modification found in the genomic DNA of diverse organisms ranging from bacteriophages to mammals.
Properties of 5-Hydroxymethyluracil
| Melting point: | >300 °C(lit.) |
| Density | 1.401±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated), Water (Slightly, Heated) |
| form | Solid |
| pka | 8.46±0.10(Predicted) |
| color | White |
| Water Solubility | Soluble in water. (50 g/L ) at 20°C, DMSO, dimethyl formamide or 100% ethanol. |
| BRN | 125482 |
| InChI | InChI=1S/C5H6N2O3/c8-2-3-1-6-5(10)7-4(3)9/h1,8H,2H2,(H2,6,7,9,10) |
| CAS DataBase Reference | 4433-40-3(CAS DataBase Reference) |
| EPA Substance Registry System | 2,4(1H,3H)-Pyrimidinedione, 5-(hydroxymethyl)- (4433-40-3) |
Safety information for 5-Hydroxymethyluracil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5-Hydroxymethyluracil
| InChIKey | JDBGXEHEIRGOBU-UHFFFAOYSA-N |
| SMILES | C1(=O)NC=C(CO)C(=O)N1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
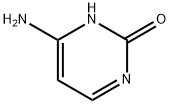
 5-(Hydroxymethyl)uracil CAS 4433-40-3View Details
5-(Hydroxymethyl)uracil CAS 4433-40-3View Details
4433-40-3 -
 5-(Hydroxymethyl)uracil CAS 4433-40-3View Details
5-(Hydroxymethyl)uracil CAS 4433-40-3View Details
4433-40-3 -
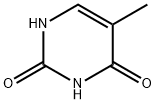
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
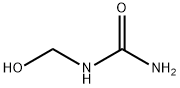
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
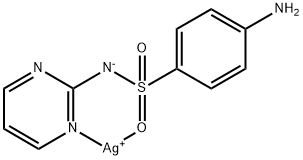
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1