5-Fluorocytosine
Synonym(s):4-amino-5-fluoro-2(1H)-pyrimidinone;5-Fluorocytosine;Flucytosine
- CAS NO.:2022-85-7
- Empirical Formula: C4H4FN3O
- Molecular Weight: 129.09
- MDL number: MFCD00006035
- EINECS: 217-968-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-12 11:51:25
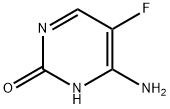
What is 5-Fluorocytosine?
Absorption
Rapidly and virtually completely absorbed following oral administration. Bioavailability 78% to 89%.
Toxicity
Oral, rat: LD50 = >15 gm/kg.
Description
5-
Description
Flucytosine is a 60-year-old medication used for treating fungal infections in humans and animals. It was?synthesized?by Robert Duschinsky and Edward Pleven at Hoffmann-La Roche (Nutley, NJ), along with Charles Heidelberger at the University of Wisconsin Medical School (Madison) in 1957. In the following few years, Duschinsky and Heidelberger obtained three US patents on the compound and its synthesis that were assigned to Hoffmann-La Roche.
Flucytosine is still in use today, as an oral or injectable medication, usually in combination with other drugs such as amphotericin B. Because the US Food and Drug Administration has approved only one flucytosine supplier (Valeant), it can cost as much as US$2,000 per day for common dosages. It is used only for serious?Candida?infections.
Since the 1980s, the flucytosine–amphotericin B combination has been used to treat HIV/AIDS-related fungal infections. The high price of flucytosine drove six researchers in a consortium of organizations (Durham University [UK], Sanofi [Gentilly and Montpellier, France], and the French nonprofit MEPI [Toulouse]) to develop a less expensive method for making the drug. They replaced the current four-step synthesis with a?one-step, continuous-flow cytosine fluorination process. Their pilot plant produces 60 g of flucytosine per hour.
Chemical properties
White Crystalline Solid
Originator
Ancobon,Roche,US,1972
The Uses of 5-Fluorocytosine
5-Fluorocytosine acts as an antidiabetic, antifungal and antimicrobial agent. It is useful for the treatment of serious infections arises due to susceptible strains of Candida or Cryptococcus neoformans and chromomycosis. Further, it is employed in studies on TMP biosynthesis.
The Uses of 5-Fluorocytosine
5-FC is a toxic antifungal/antimicrobial agent
Background
A fluorinated cytosine analog that is used as an antifungal agent.
Indications
For the treatment (in combination with amphotericin B) of serious infections caused by susceptible strains of Candida (septicemia, endocarditis and urinary system infections) and/or Cryptococcus (meningitis and pulmonary infections).
What are the applications of Application
5-Fluoro Cytosine is a toxic antimicrobial and antifungal agent
Definition
ChEBI: Flucytosine is an organofluorine compound that is cytosine that is substituted at position 5 by a fluorine. A prodrug for the antifungal 5-fluorouracil, it is used for the treatment of systemic fungal infections. It has a role as a prodrug. It is an organofluorine compound, a pyrimidone, an aminopyrimidine, a nucleoside analogue and a pyrimidine antifungal drug. It is functionally related to a cytosine.
Indications
Flucytosine (Ancobon) is a synthetic, fluorinated pyrimidine that is structurally related to fluorouracil (FU) and floxuridine. It can be fungistatic and fungicidal. Although it is used more frequently in the treatment of systemic infections caused by Candida and Cryptococcus, dermatologic indications may include infections due to chromomycosis, sporotrichosis, Cladosporium, and Sporothrix species. It is generally ineffective against Aspergillus species.
Manufacturing Process
The preparation of 5-fluorouracil is given under "Fluorouracil." As described in
US Patent 3,040,026, 5-fluorouracil is then subjected to the following steps to
give flucytosine.
Step 1: 2,4-Dichloro-5-Fluoropyrimidine - A mixture of 104 grams (0.8 mol)
of 5-fluorouracil, 1,472 grams (9.6 mols) of phosphorus oxychloride and 166
grams (1.37 mols) of dimethylaniline was stirred under reflux for 2 hours.
After cooling to room temperature, phosphorus oxychloride was removed by
distillation at 18 to 22 mm and 22° to 37°C. The residue was then poured into
a vigorously stirred mixture of 500 ml of ether and 500 gram of ice. After
separating the ether layer, the aqueous layer was extracted with 500 ml, then
200 ml of ether. The combined ether fractions were dried over sodium sulfate,
filtered, and the ether removed by vacuum distillation at 10° to 22°C. The
residue, a yellow solid melting at 37° to 38°C, weighed 120 grams
corresponding to a 90% yield. Vacuum distillation of 115 grams of this
material at 74° to 80°C (16 mm) gave 108 grams of white solid melting at
38° to 39°C corresponding to an 84.5% yield.
Step 2: 2-Chloro-4-Amino-5-Fluoropyrimidine - To a solution of 10.0 grams
(0.06 mol) of 2,4-dichloro-5-fluoropyrimidine in 100 ml of ethanol, 25 ml of
concentrated aqueous ammonia were slowly added. A slightly opalescent
solution resulted. The temperature gradually rose to 35°C. The solution was
then cooled in ice to 18°C and thereafter remained below 30°C. After three
hours, a Volhard titration showed that 0.0545 mol of chlorine was present in
ionic form. Storage in a refrigerator overnight resulted in some crystallization
of ammonium chloride. A white sludge, resulting from the evaporation of the
reaction mixture at 40°C, was slurried with 75 ml of water, filtered and
washed free of chloride. After drying in vacuo, the product melted at 196.5°
to 197.5°C, yield 6.44 grams. Evaporation of the mother liquors yielded a
second crop of 0.38 gram, raising the total yield to 6.82 grams (79.3%).
Step 3: 5-Fluorocytosine - A slurry of 34.0 grams (0.231 mol) of 2-chloro-4-
amino-5-fluoropyrimidine in 231 ml of concentrated hydrochloric acid was
heated in a water bath at 93° to 95°C for 125 minutes. The reaction was
followed by means of ultraviolet spectrophotometry using the absorption at
245, 285, and 300 mμ as a guide. The absorption at 300 mμ rose to a
maximum after 120 minutes and then dropped slightly. The clear solution was
cooled to 25°C in an ice bath, then evaporated to dryness under vacuum at
40°C. After slurrying with water three times and reevaporating, the residue
was dissolved in 100 milliliters of water. To this solution, cooled in ice, 29 ml
of concentrated ammonia were added dropwise. The resulting precipitate was
filtered, washed free of chloride with water, then with alcohol and ether. After
drying in vacuo at 65°C, the product weighed 22.3 grams. An additional 6.35
grams was obtained by evaporation of the mother liquor, thus yielding a total
of 28.65 grams (96.0%).
brand name
Ancobon (Valeant).
Therapeutic Function
Antifungal
Antimicrobial activity
The spectrum of activity is restricted to Candida spp., Cryptococcus spp. and some fungi causing chromoblastomycosis.
Acquired resistance
About 2–3 of Candida spp. isolates (more in some centers) are resistant before treatment starts, and resistance may develop during treatment. The most common cause of resistance appears to be loss of the enzyme uridine monophosphate pyrophosphorylase.
Pharmaceutical Applications
A synthetic fluorinated pyrimidine available for intravenous infusion or oral administration.
Biochem/physiol Actions
Nucleoside analog that has antifungal activities. 5-FC is deaminated by cytosine deaminase to product 5-fluorouracil, resulting in RNA miscoding. 5-Fluorocytosine inhibits DNA and RNA synthesis and interferes with ribosomal protein synthesis.
Mechanism of action
Flucytosine (5-flucytosine, 5-FC; Ancoban) is a fluorinated pyrimidine analogue of cytosine that was originally synthesized for possible use as an antineoplastic agent. It is indicated only for the treatment of serious systemic infections caused by susceptible strains of Candida and Cryptococcus spp.The mechanism of action of 5-fluorocytosine (5-FC)has been studied in detail.The drug enters the fungal cell by active transport onATPases that normally transport pyrimidines. Once insidethe cell, 5-fluorocytosine is deaminated in a reaction catalyzedby cytosine deaminase to yield 5-fluorouracil(5-FU). 5-Fluorouracil is the active metabolite of the drug.5-Fluorouracil enters into pathways of both ribonucleotideand deoxyribonucleotide synthesis. The fluororibonucleotidetriphosphates are incorporated into RNA, causingfaulty RNA synthesis. This pathway causes cell death. Inthe deoxyribonucleotide series, 5-fluorodeoxyuridinemonophosphate (F-dUMP) binds to 5,10-methylenetetrahydrofolicacid, interrupting the one-carbon pool substratethat feeds thymidylate synthesis. Hence, DNA synthesisis blocked.
Pharmacokinetics
Flucytosine is an antimetabolite that acts as an antifungal agent with in vitro and in vivo activity against Candida and Cryptococcus. Flucytosine enters the fungal cell via cytosine permease; thus, flucytosine is metabolized to 5-fluorouracil within fungal organisms. The 5-fluorouracil is extensively incorporated into fungal RNA and inhibits synthesis of both DNA and RNA. The result is unbalanced growth and death of the fungal organism. Antifungal synergism between Ancobon and polyene antibiotics, particularly amphotericin B, has been reported.
Pharmacokinetics
Oral absorption: Complete
Cmax 25 mg/kg 6-hourly oral: 70–80 mg/L after 1–2 h
Plasma half-life: 3–6 h
Volume of distribution: 0.7–1 L/kg
Plasma protein binding c. 12%
Absorption is slower in persons with impaired renal function,
but peak concentrations are higher. Levels in the CSF
are around 75% of the simultaneous serum concentration.
More than 90% of a dose of flucytosine is excreted in the
urine in unchanged form. The serum half-life is much longer
in renal failure, necessitating modification of the dosage regimen:
for patients with a creatinine clearance below 40 mL/
min the dosage interval should be doubled to 12 h; in severe
renal failure the dosage interval should be further increased to
once daily or less, based on frequent serum drug concentration
measurements.
Pharmacology
5-FC is well absorbed orally, with greater than 90% bioavailability. The serum half-life is 3 to 5 hours, with serum levels peaking 4 to 6 hours after a single dose.The drug is widely distributed in body fluids, with cerebrospinal fluid levels 60 to 80% of serum levels.The drug also penetrates well into urine, aqueous humor, and bronchial secretions.Minimal serum protein binding allows more than 90% of each dose to be excreted in the urine; significant dosage reductions are required in the presence of renal impairment. 5-FC can be removed by both hemodialysis and peritoneal dialysis. 5-FC conversion to toxic metabolites may occur in mammalian cells to a limited extent, which accounts for 5-FC toxicity.
Clinical Use
Candidosis (in combination with amphotericin B or fluconazole)
Cryptococcosis (in combination with amphotericin B or fluconazole)
Monitoring of flucytosine concentrations is desirable in all
patients, and mandatory in those with renal impairment.
Clinical Use
Flucytosine has significant antifungal activity against C. albicans, other Candida spp., C. neoformans, and the fungal organisms responsible for chromomycosis. Not considered the drug of choice for these fungal infections, 5-FC does remain useful as part of combination therapy for systemic candidiasis and cryptococcal meningitis and as an alternative drug for chromomycosis. When it is used as monotherapy, resistance and clinical failure are common. Potential mechanisms for drug resistance include decreased fungal cell membrane permeability and reduced levels of fungal cytosine deaminase. Combination therapy with amphotericin B and flucytosine in the treatment of cryptococcal meningitis and deep-seated Candida infections, such as septic arthritis and meningitis, permits reduced dosing of amphotericin B and prevents the emergence of 5-FC resistance. When higher doses of amphotericin B are used, combination therapy with 5-FC confers no additional clinical benefit except in the treatment of Candida endophthalmitis, where tissue penetration remains problematic.
Side Effects
Nausea, vomiting, abdominal pain and diarrhea are common.
Serious side effects include myelosuppression and hepatic
toxicity; they occur more frequently when serum concentrations
exceed 100 mg/L.
The nephrotoxic effects of amphotericin B can result in
elevated blood concentrations of flucytosine, and levels of the
latter drug should be monitored when these compounds are
administered together. When 5-FC is prescribed alone to patients with normal renal function, skin rash, epigastric distress, diarrhea, and liver enzyme elevations can occur.
Synthesis
Flucytosine, 5-fluorocytosine (35.4.4), is synthesized from fluorouracil (30.1.3.3). Fluorouracil is reacted with phosphorous oxychloride in dimethylaniline to make 2,4-dichloro-5-fluoropyrimidine (35.4.2), which is reacted with ammonia to make a product substituted with chlorine at the fourth position of the pyrimidine ring—4-amino- 2-chloro-5-fluoropyrimidine (35.4.3). Hydrolysis of the chlorovinyl fragment of this compound in a solution of hydrochloric acid gives the desired flucytosine.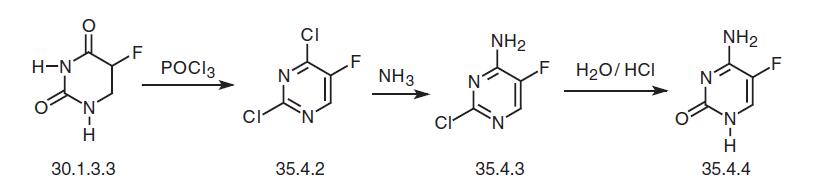
An alternative way of synthesis consists of making flucytosine from a precursor of fluorouracil—5-fluoro-2-methylthiouracil (30.1.3.2) using a somewhat analogous scheme. Treating 5-fluoro-2-methylthiouracil (30.1.3.2) with phosphorous pentachloride gives 4-chloro-5-fluoro-2-methylthiopyrimidine (35.4.5), which upon being reacted with ammonium is transformed into 4-amino-5-fluoro-2-methylthiopyrimidine (35.4.6). Hydrolysis of the methylthiovinyl fragment using concentrated hydrobromic acid gives the desired flucytosine.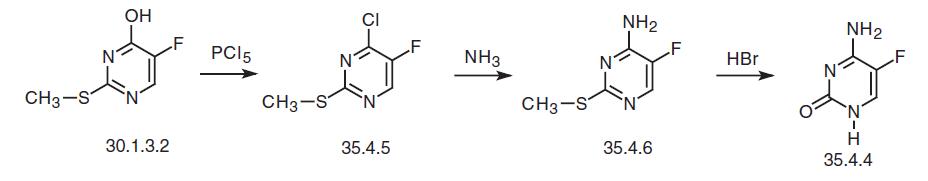
Drug interactions
Potentially hazardous interactions with other drugs Cytarabine: concentration of flucytosine possibly reduced.
Metabolism
Flucytosine is deaminated, possibly by gut bacteria or by the fungal targets, to 5-fluorouracil, the active metabolite.
Metabolism
Flucytosine itself is not cytotoxic but, rather, is a pro-drug that is taken up by fungi and metabolized to 5-fluorouracil (5-FU) by fungal cytidine deaminase. Then, 5-FU is converted to 5-fluorodeoxyuridine, which as a thymidylate synthase inhibitor interferes with both protein and RNA biosynthesis. 5-Fluorouracil is cytotoxic and is employed in cancer chemotherapy. Human cells do not contain cytosine deaminase and, therefore, do not convert flucytosine to 5-FU. Some intestinal flora, however, do convert the drug to 5-FU, so human toxicity does result from this metabolism.
Properties of 5-Fluorocytosine
| Melting point: | 298-300 °C (dec.) (lit.) |
| Density | 1.3990 (estimate) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble in water, slightly soluble in ethanol (96 per cent) |
| form | Crystalline Powder |
| pka | 3.26(at 25℃) |
| color | White to almost white |
| Water Solubility | 1.5g/100mL (25 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,4125 |
| BRN | 127285 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 2022-85-7(CAS DataBase Reference) |
Safety information for 5-Fluorocytosine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 5-Fluorocytosine
| InChIKey | XRECTZIEBJDKEO-UHFFFAOYSA-N |
5-Fluorocytosine manufacturer
JSK Chemicals
Innovative
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 2022-85-7 98%View Details
2022-85-7 98%View Details
2022-85-7 -
 5-Fluoro Cytosine 98%View Details
5-Fluoro Cytosine 98%View Details
2022-85-7 -
 2022-85-7 Flucytosine 98%View Details
2022-85-7 Flucytosine 98%View Details
2022-85-7 -
 5-Fluorocytosine CAS 2022-85-7View Details
5-Fluorocytosine CAS 2022-85-7View Details
2022-85-7 -
 5-Fluoro cytosine CAS 2022-85-7View Details
5-Fluoro cytosine CAS 2022-85-7View Details
2022-85-7 -
 5-Fluorocytosine CAS 2022-85-7View Details
5-Fluorocytosine CAS 2022-85-7View Details
2022-85-7 -
 Flucytosine CAS 2022-85-7View Details
Flucytosine CAS 2022-85-7View Details
2022-85-7 -
 Powder White 5-Fluorocytosine (CAS Number: 2022-85-7), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
Powder White 5-Fluorocytosine (CAS Number: 2022-85-7), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
2022-85-7
