Uracil
Synonym(s):2,4(1H,3H)-Pyrimidinedione;2,4-Dihydroxypyrimidine;2,4-Pyrimidinediol;Uracil
- CAS NO.:66-22-8
- Empirical Formula: C4H4N2O2
- Molecular Weight: 112.09
- MDL number: MFCD00006016
- EINECS: 200-621-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-25 18:47:31
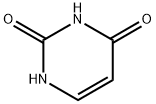
What is Uracil?
Description
Uracil is a pyrimidine base that plays a fundamental role in RNA. It pairs with adenine through hydrogen bonds. The conversion of uracil to its nucleoside form, uridine, occurs with the addition of a ribose group. Further, uridine is transformed into uridine monophosphate, a nucleotide, by the attachment of a phosphate group.
Chemical properties
Crystalline needles. Soluble in hot water, ammonium hydroxide, and other alkalies; insoluble in alcohol and ether.
The Uses of Uracil
Uracil (Lamivudine EP Impurity F) is a nitrogenous base on RNA nucleosides.
The Uses of Uracil
In biochemical research.
What are the applications of Application
Uracil is a pyrimidine derivative
Definition
Uracil is a pyrimidine nucleobase characterized by the presence of two oxo groups at the 2nd and 4th positions on its pyrimidine ring. It is a common and naturally occurring compound that plays a significant role in RNA, where it base pairs with adenine. During DNA transcription, uracil replaces thymine, serving as a nitrogenous base in RNA with a pyrimidine ring structure.
Uracil is also a key component of nucleotides and contributes to the structure of the nucleic acid RNA. As a pyrimidine derivative, it is one of the major base components of RNA.
General Description
Uracil is a nucleotide base that is integral to RNA. It serves as the precursor to thymine, which is found in DNA. In the human body, when nucleotides are produced, uracil is incorporated into a ribonucleoside triphosphate. This process involves uracil being linked with a ribose sugar and three phosphate groups, acting as a carrier of genetic information.
Safety Profile
Moderately toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Uracil crystallises from water (m 339-341o) and m 338o after sublimation in high vacuum. Its solubility in H2O at 20o is 1g/300mL. [Beilstein 24 H 312, 24 I 312, 24 II 169, 24 III/IV 1193.]
Properties of Uracil
| Melting point: | >300 °C (lit.) |
| Boiling point: | 209.98°C (rough estimate) |
| Density | 1.4421 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| storage temp. | 2-8°C |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, |
| form | Crystalline Powder |
| pka | 9.45(at 25℃) |
| color | White to slightly yellow |
| Water Solubility | SOLUBLE IN HOT WATER |
| Merck | 14,9850 |
| BRN | 606623 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 66-22-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Uracil(66-22-8) |
| EPA Substance Registry System | Uracil (66-22-8) |
Safety information for Uracil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Uracil
| InChIKey | ISAKRJDGNUQOIC-UHFFFAOYSA-N |
Uracil manufacturer
Allmpus Laboratories Pvt Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 URACIL 99%View Details
URACIL 99%View Details -
 Uracil 98% CAS 66-22-8View Details
Uracil 98% CAS 66-22-8View Details
66-22-8 -
 Uracil CAS 66-22-8View Details
Uracil CAS 66-22-8View Details
66-22-8 -
 Uracil CAS 66-22-8View Details
Uracil CAS 66-22-8View Details
66-22-8 -
 Uracil CAS 66-22-8View Details
Uracil CAS 66-22-8View Details
66-22-8 -
 Uracil CAS 66-22-8View Details
Uracil CAS 66-22-8View Details
66-22-8 -
 Uracil CAS 66-22-8View Details
Uracil CAS 66-22-8View Details
66-22-8 -
 Uracil 99% CASView Details
Uracil 99% CASView Details
