Cytosine
Synonym(s):4-Amino-2-hydroxypyrimidine;4-Aminopyrimidin-2-(1H)-one;Cytosine
- CAS NO.:71-30-7
- Empirical Formula: C4H5N3O
- Molecular Weight: 111.1
- MDL number: MFCD00006034
- EINECS: 200-749-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
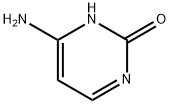
What is Cytosine?
Description
Cytosine is a pyrimidine derivative; along with adenine and guanine they account for the fi ve nucleic acid bases. Pyrimidines are heterocyclic single-ringed compounds based on the structure of pyrimidine. It is one of the principal componentbases of nucleotides and the nucleicacids DNA and RNA. When the bases combine with ribose, a ribonucleoside forms; and when it attaches to deoxyribose, a deoxyribosenucleoside is formed.
Chemical properties
White Solid
History
Cytosine is first isolated by hydrolysis of calf thymus tissue by Albrecht Kossel (1853–1927) and A. Neumann during 1893–1894. Thymine’s structure was published in 1900 and confi rmed over the next several years when it was synthesized by several investigators. In 1903, cytosine was synthesized by Henry Lord Wheeler (1867–1914) and Treat B. Johnson, confirming its structure. Uracil was first isolated in 1900 from yeast nucleic acid found in bovine thymus and herring sperm.the methylation of uracil produces thymine; thymine is also called 5-methyluracil because methylation takes place at the fi fth carbon in uracil to produce thymine.
The Uses of Cytosine
Cytosine is a nucleoside used for proteomics research. It is also used as an enzyme substrate or precursor of effector molecules such as cytosine sugars.
Biological Functions
Cytosine can also be methylated by adding a methyl (CH3) group at the C5 position and, in this modified form, plays a vital role in epigenetics. Moreover, cytosine can be transformed into other bases, such as uracil, further elevating the importance of this nitrogenous base in epigenetics. In its epigenetically modified form, cytosine associates with changes in the cellular and developmental process, neuron cell development, and human tumor development. Cytosine, in the form of cytidine triphosphate (CTP), may be used as an enzyme co-factor. Transferring a phosphate can convert adenosine diphosphate (ADP) to ATP. ATP is a high-energy molecule that is involved in a variety of cellular functions and essential biological reactions.
Biochem/physiol Actions
Cytosine (C) is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA).
Structure and conformation
Cytosine is a pyrimidine containing a single heterocyclic aromatic ring, a keto group at C2, and an amine group at C4. The molecule is planar in shape. Cytosine can form three hydrogen bonds with guanine. Due to these three hydrogen bonds, the cytosine-guanine base pair has an overall higher boiling point and greater bond strength than the adenine-thymine base pair. The high melting point makes the cytosine-guanine base-pair much more resistant to denaturation. The double strand of DNA breaks down into its single constituent strands due to high temperatures.
Properties of Cytosine
| Melting point: | >300 °C (lit.) |
| Boiling point: | 208.2°C (rough estimate) |
| Density | 0,48 g/cm3 |
| refractive index | 1.5000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Clear to very slightly hazy colorless to faint yellow solution at 50 mg/ml in 0.5 M HCL. |
| pka | 4.60, 12.16(at 25℃) |
| form | Crystalline Powder |
| color | White to slightly yellow |
| Water Solubility | soluble |
| Merck | 14,2795 |
| BRN | 2637 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 71-30-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2(1H)-Pyrimidinone, 4-amino-(71-30-7) |
| EPA Substance Registry System | 2(1H)-Pyrimidinone, 4-amino- (71-30-7) |
Safety information for Cytosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cytosine
| InChIKey | OPTASPLRGRRNAP-UHFFFAOYSA-N |
Cytosine manufacturer
Innovative
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cytosine 98%View Details
Cytosine 98%View Details -
 Cytosine 98% CAS 71-30-7View Details
Cytosine 98% CAS 71-30-7View Details
71-30-7 -
 Cytosine 98% CAS No: 71-30-7View Details
Cytosine 98% CAS No: 71-30-7View Details
71-30-7 -
 Powder White Cytosine (CAS Number: 71-30-7), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
Powder White Cytosine (CAS Number: 71-30-7), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
71-30-7 -
 PURE CHEMICAL GRADE CYTOSINE 99%View Details
PURE CHEMICAL GRADE CYTOSINE 99%View Details
71-30-7 -
 CHINA CYTOSINE 99 %, Technical GradeView Details
CHINA CYTOSINE 99 %, Technical GradeView Details
71-30-7 -
 Cytosine, Analytical Grade, 25 KGView Details
Cytosine, Analytical Grade, 25 KGView Details
71-30-7 -
 Cytosine . .View Details
Cytosine . .View Details
65-46-3
