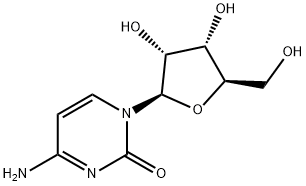
5-Aza-2'-deoxycytidine
Synonym(s):2′-Deoxy-5-azacytidine;4-Amino-1-(2-deoxy-β-D -ribofuranosyl)-1,3,5-triazin-2(1H)-one;5-Aza-CdR, 5-Aza-dC, 2′-Deoxy-5-azacytidine, Decitabine;Decitabine;InSolution
- CAS NO.:2353-33-5
- Empirical Formula: C8H12N4O4
- Molecular Weight: 228.21
- MDL number: MFCD00043011
- EINECS: 219-089-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
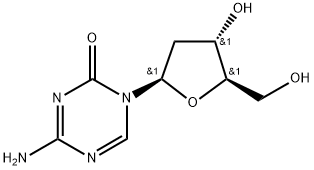
What is 5-Aza-2'-deoxycytidine?
Absorption
Decitabine administered intravenously at 15 mg/m2 for three hours every eight hours over three days resulted in a Cmax of 73.8 ng/mL (66% coefficient of variation, CV), an AUC0-∞ of 163 ng*h/mL (62% CV), and a cumulative AUC of 1332 ng*h/mL (95% CI of 1010-1730).
Similarly, decitabine at 20 mg/m2 for one hour once daily over five days resulted in a Cmax of 147 ng/mL (49% CV), an AUC0-∞ of 115 ng*h/mL (43% CV), and a cumulative AUC of 570 ng*h/mL (95% CI of 470-700).
Toxicity
Decitabine has demonstrated mutagenic potential in L5178Y mouse lymphoma cells and an Escherichia coil lac-I transgene within the colonic DNA of mice. Decitabine treatment increased chromosomal rearrangements in fruit fly larvae. In mouse models, decitabine exposure in utero (approximately 7% of the recommended daily dose) resulted in decreased weight and decreased male fertility. Adult male mice administered with between 0.3 and 1% of the recommended daily dose of decitabine three times a week for seven weeks had smaller testes with abnormal histology, decreased sperm count, and decreased fertility.
There is no known antidote for decitabine overdose. Patients experiencing an overdose are at an increased risk of severe adverse effects such as myelosuppression, including prolonged and severe neutropenia and thrombocytopenia. Symptomatic and supportive measures are recommended.
Description
Decitabine, 5-aza-2’-deoxycytidine, has been launched for the treatment of myelodysplastic syndromes (MDS). MDS are a set of hematologic disorders affecting the bone marrow that result in ineffective formation and development of blood cells. Furthermore, patients with MDS have a high risk of progressing to acute myeloid leukemia (AML). Traditional treatments include blood transfusions, hematopoietic growth factors, and prophylactic antibiotics, but these measures merely improve the quality of life with questionable effects on disease modification. While stem-cell transplantation is an aggressive, potentially curative approach, the advanced age or the other complicating health issues of most patients preclude them from considering this option. Recent advances in the underlying etiology of MDS, however, have led to the development of a new class of compounds known as ‘‘demethylating agents’’. Decitabine follows the successful introduction of the first DNA methyltransferase inhibitor azacitidine.
Chemical properties
white crystalline powder
Originator
Pharmachemie (Netherlands)
The Uses of 5-Aza-2'-deoxycytidine
Used as cancer treatment, in particular to inhibit the growth of pancreatic endocrine tumor cell lines.
The Uses of 5-Aza-2'-deoxycytidine
Decitabine is a potent inhibitor of DNA methylation with IC50 of 438 nM and 4.38 nM in HL-60 and KG1a cells, respectively
The Uses of 5-Aza-2'-deoxycytidine
5-Aza-2′-deoxycytidine has been used as a demethylating agent in breast cancer cell line, chromatin, DNA and promoter region of p16 gene.
Background
Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic neoplasms with variable underlying etiology and presentation, including neutropenia and thrombocytopenia. Further mutations leading to increased proliferation of cancerous cells can eventually lead to secondary acute myeloid leukemia, which has a poor prognosis. Among treatment options, nucleoside analogues such as decitabine and azacitidine integrate into cellular DNA and inhibit the action of DNA methyltransferases, leading to global hypomethylation and related downstream therapeutic benefits.
Decitabine was developed by MGI Pharma/SuperGen Inc. and was approved by the FDA for the treatment of MDS on February 5, 2006. It was first marketed under the name Dacogen?. It is also available as an oral combination product together with the cytidine deaminase inhibitor cedazuridine.
What are the applications of Application
5-Aza-2′-Deoxycytidine is a DNMT inhibitor shown to alter gene expression.
Indications
Decitabine is indicated for the treatment of patients with myelodysplastic syndromes (MDS) including all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia), as well as for MDS scored as belonging to the intermediate-1, intermediate-2, or high-risk group in the International Prognostic Scoring System.
Definition
ChEBI: 5-aza-2'-deoxycytidine is a 2'-deoxyribonucleoside.
brand name
Dacogen (Millot Laboratories, France).
General Description
Fine white crystalline powder. Used as a drug.
Air & Water Reactions
Probably light and air sensitive. Water soluble. Decomposes in aqueous solution at a rate that depends on pH: at pH 7 the drug is more stable than at pH 9, but is less stable than at pH 6. At pH 7 and 99°F, approximately 7% conversion occurs in 1 hour .
Reactivity Profile
5-Aza-2'-deoxycytidine is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for 5-Aza-2'-deoxycytidine are not available. 5-Aza-2'-deoxycytidine is probably combustible.
Biological Activity
Cytosine analog that once incorporated into DNA acts as a suicide substrate for DNA methyltransferase. Inhibits DNA methyltransferase and results in DNA hypomethylation and activation of silent genes. Chemotherapeutic agent; suppresses growth of human tumor cell lines. Demethylates differentiation-related genes; reverses embryonic stem cell differentiation.
Biochem/physiol Actions
Primary TargetDNA methyltransferase inhibitor
Pharmacokinetics
Decitabine is a prodrug analogue of the natural nucleotide 2’-deoxycytidine, which, upon being phosphorylated intracellularly, is incorporated into DNA and exerts numerous effects on gene expression. The use of decitabine is associated with neutropenia and thrombocytopenia. In addition, decitabine can cause fetal harm in pregnant women; effective contraception and avoidance of pregnancy are recommended during treatment with decitabine.
Clinical Use
Antineoplastic antimetabolite agent:
Treatment of acute myeloid leukaemia
Safety Profile
Poison by intravenous route.Human mutation data reported. When heated todecomposition it emits toxic fumes of NOx.
Synthesis
Silylated 5-aza-cytosine (28) was condensed with 9- fluorenylmethoxycarbonyl (Fmoc) protected 2-deoxy-1-chlororibose (27) with tin chloride (IV) in dichloroethane (Scheme 4). The coupled product 29 was de-protected with excess triethylamine in dry pyridine to give decitabine (IV) in 36% yield after separation from its corresponding |áisomer.
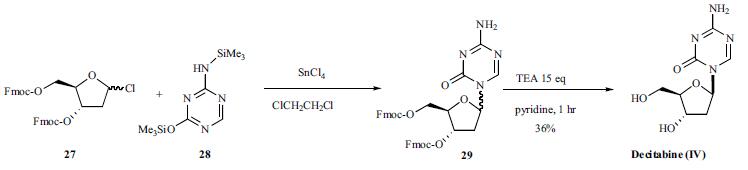
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Metabolism
Decitabine is phosphorylated inside cells by the sequential action of deoxycytidine kinase, nucleotide monophosphate kinase, and nucleotide diphosphate kinase, prior to being incorporated into newly synthesized DNA by DNA polymerase. Decitabine not incorporated into cellular DNA undergoes deamination by cytidine deaminase followed by additional degradation prior to excretion.
Metabolism
The exact route of metabolism and elimination is unknown but thought to be through deamination by cytidine deaminase in the liver, kidney, intestinal epithelium and blood to form inactive metabolites.
Storage
+4°C
References
1) Bender et al. (1998), Inhibition of DNA methylation by 5-aza-2′-deoxycytidine suppresses the growth of human tumor cell lines; Cancer Res., 58 95 2) El-Serafi et al. (2011), Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: Differential effects of 5-aza-deoxycytidine and trichostatin A; Differentiation, 81 35
Properties of 5-Aza-2'-deoxycytidine
| Melting point: | ~200 °C (dec.) |
| Boiling point: | 370.01°C (rough estimate) |
| alpha | D22 +68.5° (30 min) +57.8° (6 hr) (c = 0.5 in water) |
| Density | 1.3771 (rough estimate) |
| refractive index | 1.6590 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | acetic acid/water (1:1): 50 mg/mL |
| form | Powder |
| pka | 14.02±0.60(Predicted) |
| color | White to Off-white |
| Merck | 14,2853 |
| BRN | 617982 |
| Stability: | Stable. May be light or air sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 2353-33-5(CAS DataBase Reference) |
| EPA Substance Registry System | 5-Aza-2'-deoxycytidine (2353-33-5) |
Safety information for 5-Aza-2'-deoxycytidine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 5-Aza-2'-deoxycytidine
| InChIKey | XAUDJQYHKZQPEU-KVQBGUIXSA-N |
5-Aza-2'-deoxycytidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 2353-33-5 Decitabine 98%View Details
2353-33-5 Decitabine 98%View Details
2353-33-5 -
 Decitabine 99%View Details
Decitabine 99%View Details -
 5-Aza-2'-deoxycytidine CAS 2353-33-5View Details
5-Aza-2'-deoxycytidine CAS 2353-33-5View Details
2353-33-5 -
 Decitabine 97% CAS 2353-33-5View Details
Decitabine 97% CAS 2353-33-5View Details
2353-33-5 -
 5-Aza-2′-deoxycytidin, ≥97% CAS 2353-33-5View Details
5-Aza-2′-deoxycytidin, ≥97% CAS 2353-33-5View Details
2353-33-5 -
 Decitabine 98% (HPLC) CAS 2353-33-5View Details
Decitabine 98% (HPLC) CAS 2353-33-5View Details
2353-33-5 -
 Decitabine2353-33-5View Details
Decitabine2353-33-5View Details
2353-33-5 -
 Decitabine API 2353-33-5View Details
Decitabine API 2353-33-5View Details
2353-33-5
